
ЁОЬтФПЁПДзЫсЪЧживЊЕФвЛдЊЫсЃЌдкгаЛњКЭЮоЛњЗДгІжаЖМгагІгУЁЃЯжга25 ЁцЪБЃЌpHЃН3ЕФДзЫсЁЃЧыЛиД№вдЯТЮЪЬтЃК
ЃЈ1ЃЉШєЯђДзЫсжаМгШыЩйСПДзЫсФЦЙЬЬхЃЌДЫЪБШмвКжа![]() ________(ЬюЁАдіДѓЁБЁАМѕаЁЁБЛђЁАВЛБфЁБ)ЁЃ
________(ЬюЁАдіДѓЁБЁАМѕаЁЁБЛђЁАВЛБфЁБ)ЁЃ
ЃЈ2ЃЉШєЯђДзЫсжаМгШыЯЁNaOHШмвКЃЌЪЙЦфЧЁКУЭъШЋЗДгІЃЌЫљЕУШмвКЕФpH________(ЬюЁА>ЁБЁА<ЁБЛђЁАЃНЁБ)7ЃЌгУРызгЗНГЬЪНБэЪОЦфдвђ_____________________________________ЁЃ
ЃЈ3ЃЉШєЯђДзЫсжаМгШыpHЃН11ЕФNaOHШмвКЃЌЧвЖўепЕФЬхЛ§БШЮЊ1ЁУ1ЃЌдђЫљЕУШмвКжаИїРызгЕФЮяжЪЕФСПХЈЖШгЩДѓЕНаЁЕФЫГађЪЧ___________________________________________ЁЃ
ЃЈ4ЃЉШєЯђДзЫсжаМгШыNaOHШмвКжСШмвКЧЁКУГЪжаадЃЌДЫЪБc(NaЃЋ)______c(CH3COO-)(ЬюЁА>ЁБЁЂЁА<ЁБЛђЁАЃНЁБ)ЁЃ
ЃЈ5ЃЉШєЯђДзЫсжаМгШывЛЖЈСПNaOHШмвКЃЌЫљЕУЛьКЯвКpHЃН6ЃЌдђДЫШмвКжаc(CH3COO-)ЃC(NaЃЋ)ЃН________mol/L(ЬюаДзМШЗЪ§Он)ЁЃ
ЁОД№АИЁП МѕаЁ > CH3COOЃЃЋH2O![]() CH3COOHЃЋOHЃ cCH3COOЃ)>c(NaЃЋ)>c(HЃЋ)>c(OHЃ) ЃН 9.9ЁС10Ѓ7
CH3COOHЃЋOHЃ cCH3COOЃ)>c(NaЃЋ)>c(HЃЋ)>c(OHЃ) ЃН 9.9ЁС10Ѓ7
ЁОНтЮіЁП(1)вђДзЫсФЦЙЬЬхЕчРыВњЩњCH3COO-ЃЌc(CH3COO-)діДѓЃЌЪЙЕФЦНКтCH3COOH![]() CH3COO-+H+ФцЯђвЦЖЏЃЌc(H+)МѕаЁЃЌc(CH3COOH)діДѓЃЌЫљвд
CH3COO-+H+ФцЯђвЦЖЏЃЌc(H+)МѕаЁЃЌc(CH3COOH)діДѓЃЌЫљвд МѕаЁЃЌЙЪД№АИЮЊЃКМѕаЁЃЛ
МѕаЁЃЌЙЪД№АИЮЊЃКМѕаЁЃЛ
(2)ЯђДзЫсжаМгШыЯЁNaOHШмвКЃЌЪЙЦфЧЁКУЭъШЋЗДгІЃЌЩњГЩСЫДзЫсФЦЃЌДзЫсИљЫЎНтCH3COO-+H2O![]() CH3COOH+OH-ЃЌШмвКГЪМюадЃЌМДpHЃО7ЃЌЙЪД№АИЮЊЃКЃОЃЛCH3COO-+H2O
CH3COOH+OH-ЃЌШмвКГЪМюадЃЌМДpHЃО7ЃЌЙЪД№АИЮЊЃКЃОЃЛCH3COO-+H2O![]() CH3COOH+OH-ЃЛ
CH3COOH+OH-ЃЛ
(3)ДзЫсЙ§СПЃЌШмвКжаЕФГЩЗжЮЊДзЫсКЭДзЫсФЦЃЌвђДзЫсЕФЕчРыГЬЖШДѓгкДзЫсФЦЕФЫЎНтГЬЖШЃЌШмвКГіЫсадЃЌЫљвдc(CH3COO-)ЃОc(Na+)ЃОc(H+)ЃОc(OH-)ЃЌЙЪД№АИЮЊЃКc(CH3COO-)ЃОc(Na+)ЃОc(H+)ЃОc(OH-)ЃЛ
(4)вКЧЁКУГЪжаадЃЌдђc(H+)=c(OH-)ЃЌИљОнЕчКЩЪиКуc(Na+)+c(H+)=c(OH-)+c(CH3COO-)ЃЌЙЪc(Na+)=c(CH3COO-)ЃЌЙЪД№АИЮЊЃК=ЃЛ
(5)pH=6ШмвКжаЃЌc(H+)=10-6mol/LЃЌc(OH-)=10-8mol/LЃЌИљОнЕчКЩЪиКуc(Na+)+c(H+) =c(OH-)+c(CH3COO-)ПЩжЊЃЌc(CH3COO-)-c(Na+)=c(H+)-c(OH-) =10-6mol/L-10-8mol/L =(10-6-10-8)mol/L=9.9ЁС10Ѓ7 mol/LЃЌЙЪД№АИЮЊЃК9.9ЁС10Ѓ7mol/L


 ЬьЬьЯђЩЯвЛБОКУОэЯЕСаД№АИ
ЬьЬьЯђЩЯвЛБОКУОэЯЕСаД№АИ аЁбЇЩњ10ЗжжггІгУЬтЯЕСаД№АИ
аЁбЇЩњ10ЗжжггІгУЬтЯЕСаД№АИ
| ФъМЖ | ИпжаПЮГЬ | ФъМЖ | ГѕжаПЮГЬ |
| ИпвЛ | ИпвЛУтЗбПЮГЬЭЦМіЃЁ | ГѕвЛ | ГѕвЛУтЗбПЮГЬЭЦМіЃЁ |
| ИпЖў | ИпЖўУтЗбПЮГЬЭЦМіЃЁ | ГѕЖў | ГѕЖўУтЗбПЮГЬЭЦМіЃЁ |
| ИпШ§ | ИпШ§УтЗбПЮГЬЭЦМіЃЁ | ГѕШ§ | ГѕШ§УтЗбПЮГЬЭЦМіЃЁ |
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПЯИАћжаГЃМћЕФЛЏбЇдЊЫига20ЖржжЃЌЦфжазюЛљБОЕФдЊЫиЪЧ
A. C B. H C. O D. N
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПЯТСаИїзщЮяжЪЃЌАДЛЏКЯЮяЁЂЕЅжЪЁЂЛьКЯЮяЫГађХХСаЕФЪЧЃЈ ЃЉ
A.ЩњЪЏЛвЁЂАзСзЁЂБљЫЎЛьКЯЮя
B.ЩеМюЁЂвКбѕЁЂЕтОЦ
C.ИЩБљЁЂЬњЁЂТШЛЏЧт
D.ДПМюЁЂЕЊЦјЁЂЕЈЗЏ
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПЗАДЅУН(V2O5)ЪЧДпЛЏбѕЛЏЫљГЃгУЕФДпЛЏМСЃЌЮЊзлКЯРћгУЃЌПЦбаШЫдБзюаТбажЦСЫвЛжжРызгНЛЛЛЗЈЛиЪеЗЯЗАЕФаТЙЄвеЃЌЛиЪеТЪДя90%вдЩЯЁЃвбжЊЗЯЗАДпЛЏМСжаКЌгаV2O5ЁЂVOSO4МАВЛШмадВадќЁЃВщдФзЪСЯПЩжЊЃКVOSO4ПЩШмгкЫЎЃЌV2O5ФбШмгкЫЎЃЌNH4VO3ФбШмгкЫЎЁЃИУЙЄвеЕФСїГЬШчЯТЭМЁЃ

ЃЈ1ЃЉЫЎНўЪБЃЌЮЊСЫЬсИпЗЯЗАЕФНўГіТЪЃЌГ§СЫЗлЫщЃЌЛЙПЩвдВЩШЁЕФДыЪЉЃК___________________________________ЃЌ__________________________________ЃЈаДГі2ЬѕЃЉЃЛ
ЃЈ2ЃЉаДГіЗДгІЂйЕФРызгЗНГЬЪН_______________________________________________ЃЛ
ЃЈ3ЃЉИУЙЄвежаЗДгІЂлЕФГСЗЏТЪЪЧЛиЪеЗАЕФЙиМќЃЌГСЗАТЪЕФИпЕЭГ§ЪмШмвКpHгАЯьЭтЃЌЛЙашвЊПижЦТШЛЏяЇЯЕЪ§ЃЈNH4ClМгШыжЪСПгыСЯвКжаV2O5ЕФжЪСПБШЃЉКЭЮТЖШЁЃИљОнЯТЭМНЈвщзюКЯЪЪЕФТШЛЏяЇЯЕЪ§КЭЮТЖШЃКТШЛЏяЇЯЕЪ§ЮЊ___________ЃЌЮТЖШЮЊ_____________ЃЛ

ЃЈ4ЃЉЗДгІЂкЗЂЩњКѓЃЌШмвКжаЕФТШдЊЫивдClЃЕФаЮЪНДцдкЃЌЧыаДГіЗДгІЂкЕФЛЏбЇЗНГЬЪН__________________________________________________________________ЃЛ
ЃЈ5ЃЉВщдФзЪСЯЕУжЊЃКNH4VO3вВНаЦЋЗАЫсяЇЃЌЦфЯрЖдЗжзгСПЮЊ117ЃЌ20ЁцЪБЃЌNH4VO3ШмНтЖШЮЊ0.468gЃЌвбжЊРызгНЛЛЛКѓШмвКжаcЃЈVO3ЃЃЉ=0.10mol/LЃЌЗДгІЂлГСЗЏЪБЃЌМгШыЕШЬхЛ§0.10mol/LЕФNH4ClШмвКЃЌЭЈЙ§СаЪНМЦЫуХаЖЯДЫЪБЪЧЗёгаNH4VO3ГСЕэЮіГіЃПЃЈШмвКЬхЛ§БфЛЏКіТдВЛМЦЃЉ____________
ЃЈ6ЃЉаДГіЁАБКЩеЗДгІЂмЁБжагЩNH4VO3жЦБИV2O5ЕФЛЏбЇЗНГЬЪН__________________ЁЃ
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПЛЏКЯЮяFЪЧвЛжжгаЛњЯуОЋЕФЕїЯуМСЁЃЪЕбщЪвгЩAжЦБИFЕФвЛжжКЯГЩТЗЯпШчЯТЃК
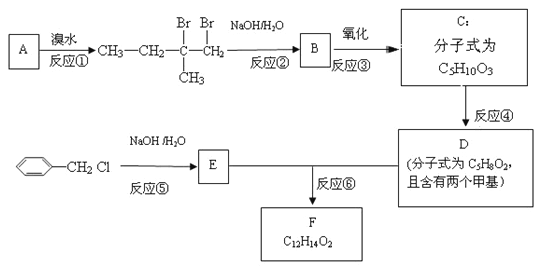
ЛиД№ЯТСаЮЪЬтЃК
ЃЈ1ЃЉЛЏКЯЮяAЕФЛЏбЇУћГЦЪЧ________________________________ЃЛ
ЃЈ2ЃЉгЩCЩњГЩDКЭDЁЂEЩњГЩFЕФЗДгІРраЭЗжБ№ЪЧ_____________ЁЂ_______________ЃЛ
ЃЈ3ЃЉCЕФНсЙЙМђЪНЮЊ________________________________________________________ЃЛ
ЃЈ4ЃЉЗДгІЂоЕФЛЏбЇЗНГЬЪНЮЊ_________________________________________________ЃЛ
ЃЈ5ЃЉЗМЯуЛЏКЯЮяGЪЧEЕФЭЌЗжвьЙЙЬхЃЌЧвGФмгыН№ЪєФЦЗДгІЗХГіH2ЃЌЦфКЫДХЙВеёЧтЦзЯдЪОга4жжВЛЭЌЛЏбЇЛЗОГЕФЧтЃЌЗхУцЛ§БШЮЊ3:2:2:1ЃЌаДГівЛжжЗћКЯвЊЧѓЕФGЕФНсЙЙМђЪНЃК_____________
ЃЈ6ЃЉаДГігУе§БћДМЃЈCH3CH2CH2OHЃЉЮЊдСЯжЦБИЛЏКЯЮяCH3COCOOCH2CH2CH3ЕФКЯГЩТЗЯп_____________________________ЃЈЦфЫќЪдМСШЮбЁЃЉЁЃ
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПЪЕбщЪвжаашвЊХфжЦ2mol/LЕФNaClШмвК950mLЃЌХфжЦЪБгІбЁгУЕФШнСПЦПЕФЙцИёКЭГЦСПЕФNaClЕФжЪСПЗжБ№ЪЧЃЈ ЃЉ
A.950mLЃЌ111.2g
B.500mLЃЌ117g
C.1000mLЃЌl17g
D.ШЮвтЙцИёЃЌ117g
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПгЩCOКЭH2SЗДгІПЩжЦЕУєЪЛљСђЃЈCOSЃЉЁЃдкКуШнЕФУмБеШнЦїжаЗЂЩњЗДгІВЂДяЕНЦНКтЃКCO(g)+H2S(g) ![]() COS(g)+H2(g)ЃЌЪ§ОнШчЯТБэЫљЪОЃК
COS(g)+H2(g)ЃЌЪ§ОнШчЯТБэЫљЪОЃК
ЪЕбщ | ЮТЖШ/Ёц | Ц№ЪМЪБ | ЦНКтЪБ | |||
n(CO)/mol | n(H2S)/mol | n(COS)/mol | n(H2)/mol | n(CO)/mol | ||
1 | 150 | 10.0 | 10.0 | 0 | 0 | 7.0 |
2 | 150 | 7.0 | 8.0 | 2.0 | 4.5 | a |
3 | 400 | 20.0 | 20.0 | 0 | 0 | 16.0 |
ЯТСаЫЕЗЈе§ШЗЕФЪЧЃЈ ЃЉ
A. ЩЯЪіЗДгІЪЧЮќШШЗДгІ
B. ЪЕбщ1ДяЦНКтЪБЃЌCOЕФзЊЛЏТЪЮЊ70%
C. ЪЕбщ2ДяЦНКтЪБЃЌa<7.0
D. ЪЕбщ3ДяЦНЦНКтКѓЃЌдйГфШы1.0molH2ЃЌЦНКтФцЯђвЦЖЏЃЌЦНКтГЃЪ§жЕдіДѓ
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПИпДПЙшОЇЬхЪЧаХЯЂММЪѕЕФживЊВФСЯЁЃ
ЃЈ1ЃЉЙЄвЕЩЯгУЪЏгЂКЭНЙЬППЩвджЦЕУДжЙшЁЃвбжЊЗДгІЙ§ГЬЕФФмСПБфЛЏШчЯТЭМ


аДГігУЪЏгЂКЭНЙЬПжЦШЁДжЙшЕФШШЛЏбЇЗНГЬЪН______________________________ЁЃ
ЃЈ2ЃЉФГЭЌбЇЩшМЦЯТСаСїГЬжЦБИИпДПЙшЃК

ЂйYЕФЛЏбЇЪНЮЊ____________________ЁЃ
ЂкаДГіЗДгІIЕФРызгЗНГЬЪН________________________________________ЁЃ
ЂлаДГіЗДгІIVЕФЛЏбЇЗНГЬЪН________________________________________ЁЃ
ЂмМзЭщЗжНтЕФЮТЖШдЖдЖИпгкЙшЭщЃЈSiH4ЃЉЃЌгУдзгНсЙЙНтЪЭЦфдвђ______________________ЁЃ
ЃЈ3ЃЉНЋДжЙшзЊЛЏГЩШ§ТШЙшЭщЃЈSiHCl3ЃЉЃЌНјвЛВНЗДгІвВПЩвджЦЕУДжЙшЁЃЦфЗДгІЃКSiHCl3(g)+H2(g)![]() Si(s)+3HCl(g)ЃЌВЛЭЌЮТЖШЯТЃЌSiHCl3ЕФЦНКтзЊЛЏТЪЫцЗДгІЮяЕФЭЖСЯБШЕФБфЛЏЙиЯЕШчЭМЫљЪОЁЃЯТСаЫЕЗЈе§ШЗЕФЪЧ__________ЃЈЬюзжФИЃЉЁЃ
Si(s)+3HCl(g)ЃЌВЛЭЌЮТЖШЯТЃЌSiHCl3ЕФЦНКтзЊЛЏТЪЫцЗДгІЮяЕФЭЖСЯБШЕФБфЛЏЙиЯЕШчЭМЫљЪОЁЃЯТСаЫЕЗЈе§ШЗЕФЪЧ__________ЃЈЬюзжФИЃЉЁЃ

AЃЎИУЗДгІЪЧЗХШШЗДгІ
BЃЎКсзјБъБэЪОЕФЭЖСЯБШПЩвдЪЧ![]()
CЃЎИУЗДгІЕФЦНКтГЃЪ§ЫцЮТЖШЩ§ИпЖјдіДѓ
DЃЎЪЕМЪЩњВњжаЮЊЬсИпSiHCl3ЕФРћгУТЪЃЌПЩвдЪЪЕБдіДѓбЙЧП
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПМјБ№ввДМЁЂБНКЭCCl4зюМђЕЅЕФЗНЗЈЪЧЃЈ ЃЉ
A. МгЫсадИпУЬЫсМиШмвККѓеёЕДЃЌОВжУ
B. гыввЫсдкгаХЈСђЫсДцдкЕФЬѕМўЯТМгШШ
C. МгеєСѓЫЎКѓе№ЕДОВжУ
D. МгЯѕЫсвјШмвККѓеёЕДОВжУ
ВщПДД№АИКЭНтЮі>>
ЙњМЪбЇаЃгХбЁ - СЗЯАВсСаБэ - ЪдЬтСаБэ
КўББЪЁЛЅСЊЭјЮЅЗЈКЭВЛСМаХЯЂОйБЈЦНЬЈ | ЭјЩЯгаКІаХЯЂОйБЈзЈЧј | ЕчаХеЉЦОйБЈзЈЧј | ЩцРњЪЗащЮожївхгаКІаХЯЂОйБЈзЈЧј | ЩцЦѓЧжШЈОйБЈзЈЧј
ЮЅЗЈКЭВЛСМаХЯЂОйБЈЕчЛАЃК027-86699610 ОйБЈгЪЯфЃК58377363@163.com