
����Ŀ���ߴ��辧������Ϣ��������Ҫ���ϡ�
��1����ҵ����ʯӢ�ͽ�̿�����Ƶôֹ衣��֪��Ӧ���̵������仯����ͼ


д����ʯӢ�ͽ�̿��ȡ�ֹ���Ȼ�ѧ����ʽ______________________________��
��2��ijͬѧ������������Ʊ��ߴ��裺

��Y�Ļ�ѧʽΪ____________________��
��д����ӦI�����ӷ���ʽ________________________________________��
��д����ӦIV�Ļ�ѧ����ʽ________________________________________��
�ܼ���ֽ���¶�ԶԶ���ڹ��飨SiH4������ԭ�ӽṹ������ԭ��______________________��
��3�����ֹ�ת�������ȹ��飨SiHCl3������һ����ӦҲ�����Ƶôֹ衣�䷴Ӧ��SiHCl3(g)+H2(g)![]() Si(s)+3HCl(g)����ͬ�¶��£�SiHCl3��ƽ��ת�����淴Ӧ���Ͷ�ϱȵı仯��ϵ��ͼ��ʾ������˵����ȷ����__________������ĸ����
Si(s)+3HCl(g)����ͬ�¶��£�SiHCl3��ƽ��ת�����淴Ӧ���Ͷ�ϱȵı仯��ϵ��ͼ��ʾ������˵����ȷ����__________������ĸ����

A���÷�Ӧ�Ƿ��ȷ�Ӧ
B���������ʾ��Ͷ�ϱȿ�����![]()
C���÷�Ӧ��ƽ�ⳣ�����¶����߶�����
D��ʵ��������Ϊ���SiHCl3�������ʣ������ʵ�����ѹǿ
���𰸡� SiO2(s)+2C(s)=Si(s)+2CO(g) H=+638.4kJ/mol H2SiO3��H4SiO4 SiO2+2OH-=SiO32-+H2O SiO2+4Mg![]() Mg2Si+2MgO ���ڱ��У�����̼����ͬ���壬ԭ�Ӱ뾶Si����C����Ԫ�صķǽ���������̼Ԫ�أ���������ȶ������ڼ��� BC
Mg2Si+2MgO ���ڱ��У�����̼����ͬ���壬ԭ�Ӱ뾶Si����C����Ԫ�صķǽ���������̼Ԫ�أ���������ȶ������ڼ��� BC
����������1����Si(s)+O2(g)==SiO2(g) ��H=-859.4kl/mol����2C(s)+ O2(g)==2CO(g) ��H=-221.0kl/mol���ɢ�-�ٿɵ�ʯӢ�ͽ�̿��ȡ�ֹ���Ȼ�ѧ����ʽ��SiO2(s)+2C(s)=Si(s)+2CO(g) H=+638.4kJ/mol����2����XΪ�����ƣ���YΪ���ᣬ��д��H2SiO3��H4SiO4���ڷ�ӦI�����ӷ���ʽΪ��SiO2+2OH-=SiO32-+H2O����þ����������ڸ��������·�Ӧ�Ļ�ѧ����ʽΪ��SiO2+4Mg![]() Mg2Si+2MgO���ܼ���ֽ���¶�ԶԶ���ڹ��飨SiH4��Դ��̼����������ļ��ܴ�С�����ڱ��У�����̼����ͬ���壬ԭ�Ӱ뾶Si����C����Ԫ�صķǽ���������̼Ԫ�أ�̼����ļ��ܴ��ڹ�����ļ��ܣ���������ȶ������ڼ��顣��3����Ͷ�ϱ�һ��ʱʱ������Ͷ�ϱ����ߣ������¶����ߣ�T1����T2����T3����SiHCl3��ƽ��ת��������˵������Ӧ�����ȷ�Ӧ����A��������������Ũ�ȿ����SiHCl3��ƽ��ת���ʣ�B��ȷ��������Ӧ�����ȷ�Ӧ�������¶ȣ�ƽ�������ƶ�����Ӧ��ƽ�ⳣ������C��ȷ�����ڷ�Ӧǰ���������С�ڷ�Ӧ�����������������ѹǿ��ƽ�������ƶ���SiHCl3��ƽ��ת���ʽ��ͣ�D����ѡBC��
Mg2Si+2MgO���ܼ���ֽ���¶�ԶԶ���ڹ��飨SiH4��Դ��̼����������ļ��ܴ�С�����ڱ��У�����̼����ͬ���壬ԭ�Ӱ뾶Si����C����Ԫ�صķǽ���������̼Ԫ�أ�̼����ļ��ܴ��ڹ�����ļ��ܣ���������ȶ������ڼ��顣��3����Ͷ�ϱ�һ��ʱʱ������Ͷ�ϱ����ߣ������¶����ߣ�T1����T2����T3����SiHCl3��ƽ��ת��������˵������Ӧ�����ȷ�Ӧ����A��������������Ũ�ȿ����SiHCl3��ƽ��ת���ʣ�B��ȷ��������Ӧ�����ȷ�Ӧ�������¶ȣ�ƽ�������ƶ�����Ӧ��ƽ�ⳣ������C��ȷ�����ڷ�Ӧǰ���������С�ڷ�Ӧ�����������������ѹǿ��ƽ�������ƶ���SiHCl3��ƽ��ת���ʽ��ͣ�D����ѡBC��


 ��ǰ�κ�ͬ����ϰϵ�д�
��ǰ�κ�ͬ����ϰϵ�д� ����С��ҵϵ�д�
����С��ҵϵ�д� �Ƹ�С״Ԫ����������ϰ��ϵ�д�
�Ƹ�С״Ԫ����������ϰ��ϵ�д� �ɹ�ѵ���ƻ�ϵ�д�
�ɹ�ѵ���ƻ�ϵ�д� ����ѵ����ֱͨ�п�����ϵ�д�
����ѵ����ֱͨ�п�����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���ס��ҡ���Ϊ�������ʡ�A��B��C��D��E��F��G��H��Ϊ��ѧ��ѧ�г����Ļ��������B��G����ɫ��Ӧ��Ϊ��ɫ��C��ʹƷ����Һ��ɫ����һ�������£��������ת����ϵ��ͼ��ʾ��
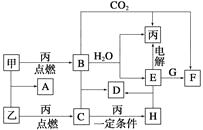
��ش��������⣺
��1���û�ѧʽ��ʾ����Ϊ__________��HΪ__________��
��2��A�ĵ���ʽΪ___________________________________________________��
��3�����E��ˮ��Һʱ��E��������_______________________________��
��4��д��B��C����D�Ļ�ѧ����ʽ��__________________________________��
д��E��G����F�����ӷ���ʽ��____________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����������Ҫ��һԪ�ᣬ���л�������Ӧ�ж���Ӧ�á�����25 ��ʱ��pH��3�Ĵ��ᡣ��ش��������⣺
��1����������м������������ƹ��壬��ʱ��Һ��![]() ________(��������������С������������)��
________(��������������������������)��
��2����������м���ϡNaOH��Һ��ʹ��ǡ����ȫ��Ӧ��������Һ��pH________(����>����<����������)7�������ӷ���ʽ��ʾ��ԭ��_____________________________________��
��3����������м���pH��11��NaOH��Һ���Ҷ��ߵ������Ϊ1��1����������Һ�и����ӵ����ʵ���Ũ���ɴ�С��˳����___________________________________________��
��4����������м���NaOH��Һ����Һǡ�ó����ԣ���ʱc(Na��)______c(CH3COO-)(����>������<����������)��
��5����������м���һ����NaOH��Һ�����û��ҺpH��6�������Һ��c(CH3COO-)��C(Na��)��________mol/L(��дȷ����)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������ʵ���о��к���ɫ����������Աȷ������ý��۲���ȷ���ǣ�������
|
|
|
�� | �� | �� |
A. �����еĺ���ɫ���壬�ƶϲ���������һ���ǻ������
B. ����ɫ���岻�ܱ�������ľ̿��Ũ��������˷�Ӧ
C. ����˵��Ũ������лӷ��ԣ����ɵĺ���ɫ����Ϊ��ԭ����
D. ������������м���CO2���ɴ�˵��ľ̿һ����Ũ���ᷢ���˷�Ӧ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����N��B��Ԫ����ɵ����Ͳ������Ź㷺��;��
��1��B2H6��һ�ָ���ȼ�ϣ�����Cl2��Ӧ���ɵ�BCl3�����ڰ뵼����ӹ��ռ��ߴ�������죻�ɵڶ�����Ԫ����ɵ���BCl3��Ϊ�ȵ������������Ϊ_______�������ӷ��ţ���һ������
��2�������飨H3N��BH3����Ti��BH4��3��Ϊ���ܹ�ע�����ͻ�ѧ�⻯�ﴢ����ϣ�
��H3N��BH3��Bԭ�ӵ���Χ�����Ų�ͼ_________��
��Ti��BH4��3��TiCl3��LiBH4��Ӧ�Ƶã�д�����Ʊ���Ӧ�Ļ�ѧ����ʽ____����̬Ti3+�ijɶԵ�����___�ԣ�BH4-�����幹����____��Ti��BH4��3������ѧ����������____��
�۰����������Ԫ��״�����HB=NH��3ͨ�����·�Ӧ�Ƶã�3CH4+2��HB=NH��3+6H2O��3CO2+6H3BNH3����������ѧ����ʽ�йص���������ȷ���� _____________
A���������д�����λ��
B����һ�����ܣ�N��O��C��B
C����Ӧǰ��̼ԭ�ӵĹ���ӻ����Ͳ���
D��CH4��H2O��CO2���ǷǼ��Է���
��3������BP�����ܵ��߶ȹ�ע����ĥ���ϣ���ͼ1Ϊ������
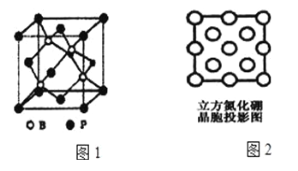
�پ�����Pԭ������Bԭ����Χ�ɵ�____��϶�С�
�ھ�����Bԭ����Χ�������ȵ�Bԭ����____����
��4��������������һ�����͵ij�Ӳ����ĥ�����µĽṹ���ϣ���ṹ��Ӳ�ȶ�����ʯ���ƣ����۵�Ƚ��ʯ�ͣ�ԭ����__________��ͼ2��������������z���ͶӰͼ������ͼ��Բ����Ϳ���ͻ��������ֱ����B��N�����λ��______�����С�����Bԭ�ӣ�����������Nԭ�ӡ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ʳ�μӵ����������������貹������������ȱ��������ƶѪ�������е����⡢�ơ�������ָ�� ��
A.����B.ԭ��C.����D.Ԫ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��X��Y��Z��M��Q��R��6�ֶ�����Ԫ�أ���ԭ�Ӱ뾶����Ҫ���ϼ����£�
Ԫ�ش��� | X | Y | Z | M | Q | R |
ԭ�Ӱ뾶/nm | 0.160 | 0.143 | 0.102 | 0.089 | 0.074 | 0.037 |
��Ҫ���ϼ� | +2 | +3 | +6��-2 | +2 | -2 | +1 |
��1��Z��Ԫ�����ڱ��е�λ����______________________________��
��2��X��Y��Q�����γɼ����ӣ��������Ӱ뾶������__________�������ӷ��ţ���
��3�����ڱ�����Щ���ڶԽ��ߣ����ϡ����£�λ�õ�Ԫ�أ����ǵĵ��ʼ��仯������������ƣ���M����������ǿ����Һ��Ӧ�����ӷ���ʽ______________________________��
��4��Q��R��ԭ�Ӹ�����1:1��ɵĻ��������һ�֡���ɫ����������
�ټ����������ۼ�������____________________��
�ڿ�������������Ʊ���װ����ͼ��ʾ���ڼ�����Һ�У����ÿ����е�������ԭ�õ���ϡ�����Һ��ͼ��a��__________���������ĵ缫��Ӧʽ��____________________��

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����14�֣�̼������������ѧ��ѧ��Ҫ�ķǽ���Ԫ�أ��ڹ�ũҵ�������й㷺��Ӧ�á�
(1�����ڷ������칬һ�����ij������Ż����ȼ����Һ̬ƫ�����£�CH3��2N��NH2����������Һ̬�����������������ڷ�Ӧ�����зų�����������ͬʱ������������Ⱦ�����塣��֪�����£�1 gȼ����ȫȼ���ͷų�������Ϊ42.5kJ����д���÷�Ӧ���Ȼ�ѧ����ʽ________________________________________��
��2��298 Kʱ����2L���ܱ������У��������淴Ӧ��
2NO2(g)![]() N2O4(g) ��H����a kJ��mol��1(a��0)
N2O4(g) ��H����a kJ��mol��1(a��0)
N2O4�����ʵ���Ũ����ʱ��仯��ͼ����ƽ��ʱ��N2O4��Ũ��ΪNO2��2�����ش��������⡣

��298kʱ���÷�Ӧ��ƽ�ⳣ��Ϊ________��
�����¶�ΪT1��T2ʱ��ƽ����ϵ��NO2�����������ѹǿ�仯������ͼ��ʾ������˵����ȷ����

a��A��C����ķ�Ӧ���ʣ�A��C
b��B��C����������ƽ����Է���������B��C
c��A��C�����������ɫ��A�Cdz
d����״̬B��״̬A�������ü��ȵķ���
������Ӧ��398K���У�ijʱ�̲��n(NO2��="0.6" mol n(N2O4��=1.2mol�����ʱV������ V���棩������>������<������=������
��3��NH4HSO4�ڷ����Լ���ҽҩ�����ӹ�ҵ����;�㷺������100 mL 0.1 mol��L��1NH4HSO4��Һ�еμ�0.1 mol��L��1NaOH��Һ���õ�����ҺpH��NaOH��Һ����Ĺ�ϵ������ͼ��ʾ��
�Է���ͼ��a��b��c��d��e����㣬
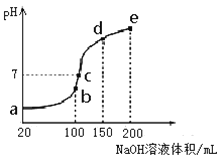
��ˮ�ĵ���̶�������__________��
������Һ��c(OH-)����ֵ��ӽ�NH3��H2O�ĵ��볣��K��ֵ���� ��
����c�㣬��Һ�и�����Ũ���ɴ�С������˳����_______��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ijС��������ͼװ�ã��ñ�������FeBr3���������Ʊ��屽��

�� | �� | �屽 | |
�ܶ�/g��cm-3 | 0.88 | 3.10 | 1.50 |
�е�/��C | 80 | 59 | 156 |
ˮ���ܽ��� | �� | �� | �� |
ʵ����̣���a�м���15mL��ˮ����������м����b��С�ļ���4.0mLҺ̬�塣��a�е��뼸���塣��Ӧ���ҽ��С���Ӧֹͣ���������̷����ᴿ��Ʒ��
 ��
��
��1����ƿ���д�������ɫ�������Թ�d�е������ǣ���______________���� ����ˮ��ɻ�ɫ��c��������___________________________________��
��2�������ᴿʱ��������Ϊ______________��������Ϊ_________________��
��3����ˮϴ����Ҫ�õ��IJ���������_________���ձ�����ˮϴ��������ˮ���еμ�KSCN��Һ����Һ���ɫ���Ʋ�ˮϴ����ҪĿ���dz�ȥ__________________��
��4����NaOH��Һϴ��ʱ��Ӧ�Ļ�ѧ����ʽ��________________________��
��5����֪�����巢������ȡ����Ӧ���ƲⷴӦ���Թ�d��Һ�庬�е����ִ���������H+��Br�������ʵ�鷽����֤�Ʋ⡣����ѡ�Լ���Mg��CCl4��AgNO3aq��H2O��
ʵ�鲽�� | Ԥ������ | ���� |
����1�����Թ�d��Һ��ת���Һ©���� __________________________________������ȡ��Һ�ȷֳ����ݣ�����A��B���Թ��У����в���2��3�� | ||
����2�� �� | ֤���� ���� | |
����3�� �� | ֤���� ���� |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com