
����Ŀ����������˵����ȷ����__________��
A��Ԫ�صĵ縺��Խ���䵥��Խ�ȶ�
B�����Ӿ����п��ܲ����ڹ��ۼ�
C��������Խ���γɵ����Ӿ���Խ�ȶ�
D��������������Ӿ����������չ��
���������к���C��N��Mn��Ԫ�أ�ʵ���г��ù��������������ⶨ�������̵ĺ�������Ӧԭ��Ϊ2Mn2++5S2O82-+8H2O ![]() 2MnO4-+10SO42-+16H+
2MnO4-+10SO42-+16H+
��1��Mnԭ�ӵļ۲���ӵĹ������ʽ�������Ų�ͼ��Ϊ____________________��
��2����֪H2S2O8�Ľṹ��ʽ��ͼ��ʾ��

��H2S2O8��S�Ĺ���ӻ���ʽΪ______________��H��O��S����Ԫ���У��縺������Ԫ����___________����Ԫ�ط��ţ���
��S��̬ԭ���е��ӵ��˶�״̬��_________�֡�
��������Ӧ��S2O82-���ѵĹ��ۼ�����Ϊ___________����������������������) ��ÿ����1mol MnO4-�����ѵĹ��ۼ���ĿΪ___________NA��

��3��C��N���γɶ��ֽṹ�ľ��塣һ�����͵ij�Ӳ���������ڽ��ʯ�Ľṹ����Ӳ�ȱȽ��ʯ���侧����ͼ��ʾ��ͼʾԭ�Ӷ������ھ����ڣ����仯ѧʽΪ______________����֪��������a=0.64nm��b=0.55nm��c=0.24nm����þ�����ܶ�Ϊ_______________���г�ʽ�Ӽ��ɣ���ʽ���в�������ĸ��g/cm3��
���𰸡� BC ![]() sp3 O 16 ���� 2.5 C3N4
sp3 O 16 ���� 2.5 C3N4 ![]()
�������������������A�������ȶ�������ӽṹ�йأ�B��ϡ�����幹�ɵķ��Ӿ����в����ڹ��ۼ���C��������Խ�����Ӽ�����Խ����D�����Ӿ��岻��������չ����
������1��Mn��25��Ԫ�أ��۵��ӵĵ����Ų�ʽ��![]() �����ݺ��ع��������������ԭ����дMn�۲���ӵĹ������ʽ��
�����ݺ��ع��������������ԭ����дMn�۲���ӵĹ������ʽ��
��2�����ݼ۲���Ӷ�=�� �����Ӷ�+����ԭ���ϵŵ��Ӷԣ����S2O8 2-�Ľṹ�ж�Sԭ���ӻ�����;�ǽ�����Խǿ�縺��Խ����
ԭ�Ӻ�����һ�����Ӿ���1���˶�״̬��
��Ӧ��S2O82-���ѵĹ��ۼ���O-O���������������������������ӷ���ʽÿ����2mol MnO4-������5mol S2O82-��
��3��ͼʾԭ�Ӷ������ھ����ڣ�����ͼʾ��ÿ����������6��Cԭ����8��Nԭ����ÿ��������������![]() ��ÿ�������������
��ÿ�������������![]() ������
������![]() �����ܶȣ�
�����ܶȣ�
��������A�������ȶ�������ӽṹ�й�����F�ĵ縺�Դ���N��N2��F2�ȶ�����A������B��ϡ�����幹�ɵķ��Ӿ����в����ڹ��ۼ�����B��ȷ��C��������Խ�����Ӽ�����Խ��������Խ�ȶ�����C��ȷ��D�����Ӿ��岻��������չ������D������
������1��Mn��25��Ԫ�أ��۵��ӵĵ����Ų�ʽ��![]() �����ݺ��ع��������������ԭ���� Mn�۲���ӵĹ������ʽ��
�����ݺ��ع��������������ԭ���� Mn�۲���ӵĹ������ʽ��![]() ��
��
��2������ԭ�Ӽ۲���Ӷ���=�� �����Ӷ�+����ԭ���ϵŵ��Ӷ�=4+12(6-4��1-2)=4������Sԭ�Ӳ�ȡsp3�ӻ����ǽ�����Խǿ�縺��Խ����H��O��S����Ԫ���У��縺������Ԫ����O��
����һ�����Ӿ���1���˶�״̬��Sԭ�Ӻ�����16�����ӣ�����S��̬ԭ���е��ӵ��˶�״̬��16����
����Ӧ��S2O82-���ѵĹ��ۼ���O-O��������������������S2O82-���ѵĹ��ۼ�����Ϊ�������������ӷ���ʽÿ����2mol MnO4-������5mol S2O82-��ÿ����1mol MnO4-�����ѵ�S2O82-�й��ۼ���ĿΪ2.5 NA��
��3��ͼʾԭ�Ӷ������ھ�����������ÿ����������6��Cԭ����8��Nԭ������ѧʽΪC3N4��ÿ��������������![]() ��ÿ�������������
��ÿ�������������![]() ������
������![]() ���ܶ�=
���ܶ�= ![]() ��
��![]() =
=![]() g/cm3��
g/cm3��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�������ʵ����� SO2��SO3���й�ϵ������ǣ� ��
A. ������ԭ�ӵ����ʵ���֮��Ϊ1:1
B. ��ԭ�ӵ����ʵ���֮��Ϊ2:3
C. ��Ԫ�ص�������Ϊ3:2
D. ��Ԫ�ص�������Ϊ1:1
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������˵������ȷ������ ��
A. �����ʶ�����C��H��N�� O����Ԫ����ɵ�
B. ������ˮ������ղ���Ϊ������
C. ǿ�ᡢǿ����ؽ����ζ���ʹ�����ʱ���
D. ���ɵ����ʵ�ijЩ���������������Dz��ܺϳɵ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�������й�ʵ������������У�����ȷ����
A. ������Cl2��ȼ�գ�������ɫ���棬ƿ���а�������
B. ��˿��Cl2��ȼ�գ������غ�ɫ����
C. ����Cl2��ȼ�գ����ɰ�ɫ����
D. ͭ˿��Cl2��ȼ�գ������ػ�ɫ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ij��ѧ��ȤС��ѡ������װ�ú�ҩƷ��ȡ������Ȳ�������й���Ȳ���ʵ�̽�����Իش��������⡣
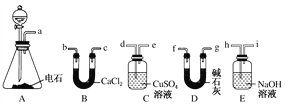
��1��A����ȡ��Ȳ�Ļ�ѧ����ʽΪ_________________________________��
��2������Ȳʱ��������Һ©���Ļ�����ʹˮ�������µ�ԭ����_________________��
��3���õ�ʯ�Ƶõ���Ȳ�г�����H2S��PH3�����ʣ���ȥ����Ӧѡ��________(����ţ���ͬ)װ�ã�������Ȳ���ѡ��________װ�á�
��4��Ϊ��̽����Ȳ��HBr�����ӳɷ�Ӧ����йز����������ʵ�飺
������Ȳ��![]() ���Һ
���Һ![]() �л��������
�л��������![]() ���Һ
���Һ![]() �л��������
��������
�ٲ���b��������________��
���л�����������ܺ��е�������________(д�ṹ��ʽ)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����������ǵ��ƾ��в�ݮ�����ѡ�ӣ�ҡ�����������ζ��ʳ���㾫�����ڷ�����ϴ�Ӽ�����ζ�����ĵ�ζ����ҽҩ��ҵ����Ϊ�л��ϳɵ��м��塣
��1������������C��H��O����Ԫ����ɣ�����������ӵ���Է�������Ϊ162���˴Ź���������ͼ��ʾ��6���壬�����֮��Ϊ1�U2�U2�U1�U1�U3 �����ú�������Ǽ�����е�ijЩ���ţ���ú����������ͼ��
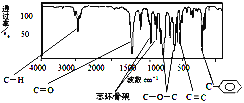
�����������Ľṹ��ʽ��_____________ (�����������칹)��
��2����֪��I��ȩ��ȩ�ܷ�����Ӧ��ԭ�����£�
![]()
II����֪��A�ڱ�״���µ��ܶ�Ϊ1.25g��L-1���ϳ����������Ĺ�ҵ��������ͼ��ʾ��
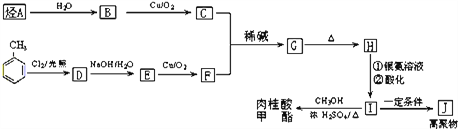
��ش�
��������J�Ľṹ��ʽΪ_______________��
��������G�еĹ�������______________��
��G��HΪ_______________��Ӧ(�Ӧ����)��
��д����ӦD��E�Ļ�ѧ����ʽ______________________��
����������������I��ͬ���칹�干��5�֡�д��������ͬ���칹��Ľṹ��ʽ��_____________
A���ܷ���ˮ�ⷴӦ B����������Һ���ó��ֹ���������C�������巢���ӳɷ�Ӧ
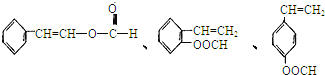
![]()
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����֪�ױ����Խ�������ת����

�ش��������⣺
��1����Ӧ���ķ�Ӧ����Ϊ__________����Ӧ���ķ�Ӧ����Ϊ_____________��
��2��������A�Ľṹ��ʽΪ____________��������D�ķ���ʽΪ_____________��
��3����Ӧ���Ļ�ѧ����ʽΪ_______________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������ҩ�����������ڹ���ʹ�õ�������������ҩ���õ�Σ������ (����)��
A���г�ҩ B�������� C������ҩ D��������ʹҩ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����1����֪����Cl2ͨ������KOH��Һ�������п�����KC1��KClO��KC1O3����c(Cl-):c(ClO-)��ֵ���¶ȸߵ��йء���n(KOH)=amolʱ����ij�¶��£���Ӧ��c(Cl-):c(ClO-)��11������Һ��c(ClO-):c(ClO3-)��_________________
��2����P��CuSO4��H2O��Cu3P��H3PO4��H2SO4(δ��ƽ)�ķ�Ӧ�У�7.5molCuSO4������P�����ʵ���Ϊ________mol������1molCu3Pʱ���μӷ�Ӧ��P�����ʵ���Ϊ________mol��
��3��һ������CuS��Cu2S�Ļ����Ͷ��������HNO3�У��ռ�������VL(��״��)����Ӧ�����Һ�У�����Cu2����SO![]() ����������NaOH��������ɫ���������ˣ�ϴ�ӣ����գ��õ�CuO 16.0g������������ΪNO��NO2�Ļ����������Ϊ1��1����V�ļ�СֵΪ________mL��
����������NaOH��������ɫ���������ˣ�ϴ�ӣ����գ��õ�CuO 16.0g������������ΪNO��NO2�Ļ����������Ϊ1��1����V�ļ�СֵΪ________mL��
��4����һ������Fe��FeO��Fe3O4�Ļ�����У�����1 mol/L �������Һ100 mL��ǡ��ʹ�����ȫ���ܽ⣬�ҷų�336 mL����״���£������壬��������Һ�м���KSCN��Һ����Һ��ɫ���֣���ȡ��ͬ������Fe��FeO��Fe3O4�Ļ�������1 mol/L��ϡ������Һ��Ҳǡ��ʹ�������ȫ�ܽ⣨���軹ԭ����Ψһ������Ӧ����������Һ�м���KSCN��Һ����ҺҲ��ɫ���֣���������ĵ�ϡ����������__________mL��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com