
��15�֣�ij��ѧ��ȤС���ú�����������ͭ�ĺϽ���ȡ�������Ȼ�����Һ���̷�����͵������壬��̽����ҵ���ϵ������á���ʵ�鷽�����£�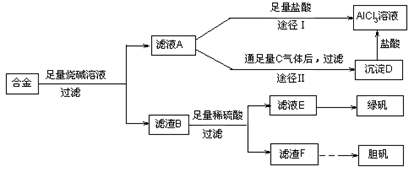
��ش���������:
��1������ҺA�Ƶ�AlCl3��Һ��;����͢�����������Ϊ�������� ��������:
��
��2������ҺE�еõ��̷������ʵ������� ��
��3������һ���ܷ�Ӧʽ��ʾ������F�Ʊ�������������з����ı仯:
��
��4����ͬѧ����ɽ�����������ܽ�Ͻ���ռ�������ᣬ������Ʒ�����Ҳ���Ƶ��������ʣ�����Ϊ���ߵķ����Ƿ������ ��������
��
��1��;�������������Ϊ��ҺA��NaAlO2��Һ����;��Iֱ����A��������õ���AlCl3��Һ�к��д�����NaCl���ʣ���;��II��ͨ��c��CO2�����壬��D[Al(OH)3]��������Al(OH)3�ܽ��������еõ����Ǵ�����AlCl3��Һ������;��II��������2������Ũ������ȴ�ᾧ��ϴ�ӡ����3��2Cu + O2 + 2H2SO4+8H2O 2CuSO4��5H2O ��4�����ߵķ��������� ��Ϊ�÷���������ʵ�鷽����Ƶļ�Լ��ԭ����������ࡢ�����Լ�������ʱ�䳤��
2CuSO4��5H2O ��4�����ߵķ��������� ��Ϊ�÷���������ʵ�鷽����Ƶļ�Լ��ԭ����������ࡢ�����Լ�������ʱ�䳤��
���������������1��ƫ�����ƺ��������ᷴӦ�����Ȼ������Ȼ��ƵĻ�������ƫ��������ͨ�������Ķ�����̼����õ������������������������������ܽ⣬������Һ�ijɷ�ֻ���Ȼ������ʴ�Ϊ������Ϊ��ҺA��ƫ�����ƺ�����������Һ����;��Iֱ����A�м�������õ���AlCl3��Һ�к��д������Ȼ������ʣ���;��IIͨ�������̼���壬��Al(OH)3��������Al(OH)3�ܽ��������еõ����Ǵ�����AlCl3��Һ����2�������Һ���Ʊ��̷�ʱӦ�Ƚ���Һ����Ũ����Ȼ����ȴ�ᾧ��ϴ�Ӹ���ɵõ��̷����ʴ�Ϊ������Ũ������ȴ�ᾧ��ϴ�ӡ������3���ɿ����Ʊ��������ʿ���Ϊͭ��ͭ���ܺ�ϡ���ᷴӦ�������Ժ����ᷴӦ����������F�ǽ���ͭ��Cu�м���ϡ��������Ʊ��������壬��Ӧ����ʽΪ��2Cu+2H2SO4+8H2O+O2=2CuSO4?5H2O����4����ʵ�鷽��һ�У�������Ŀ�����û�й��ߵ�Ҫ��ֻ��������ɣ����Ƿ������У���Ҫ�����������ᣬ����ѿأ��ʴ�Ϊ������������Ϊ�÷�����������ࡢʱ�䳤�������Լ�����
���㣺��������Ԫ�صĵ��ʼ��仯������ۺ�Ӧ�ã��Ʊ�ʵ�鷽�������



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
ijͬѧ��̽��Ũ���ᡢϡ���ᡢŨ���ᡢϡ����ֱ�������ͭ��Ӧ��ʵ���е��й����⡣
��1���ڳ����£��ֱ���ʢ�е�����Ƭ����֧�Թ��м��������Ģ�Ũ���� ��ϡ���� ��Ũ���� ��ϡ���ᣬ�ܹ۲쵽�������Է�Ӧ���ǣ�����ţ���ͬ�� �������Է�Ӧ������� ��
��2���ֱ���ʢ�е���ͭƬ����֧�Թ��м��������Ģ�Ũ���� ��ϡ���� ��Ũ���� ��ϡ���ᣬ�ܹ۲쵽�漴�������ҷ�Ӧ���� ����ʼ����Ӧ�����Ⱥ�����Ӧ���� ������Ҳ��������Ӧ���� ���ܻ���������Ӧ����ʱ�ܷ�����Ӧ���� ����Ӧ�����ӷ���ʽΪ�� ;
�Ƚ�ͭ��Ũ���ᷴӦ����������X����ͨ����ͼװ���У�һ��ʱ����ٽ�ͭ��Ũ���ᷴӦ�����Ĵ�������YҲ����ͨ���װ���У��ɹ۲쵽��������� ��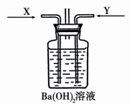
a��ͨ��X������Ȳ�����ɫ�������ܽ�
b��ͨ��X�������Һ������������
c��ͨ��Y������г�������
d��ͨ��Y���������ܽ�
e��ͨ��Y�������Һ������������
��3��������2����Ba��OH)2��Ϊ�����ᣬ��ͨ��X����������Ϊ ����Ӧ�Ļ�ѧ����ʽΪ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
����ʯ����ȡ�طʺ�������������Ҫԭ�ϣ�����ʯ����ɺ��������ƣ�����������������������������ʡ�����ʵ�鲽������ͼ��ʾ��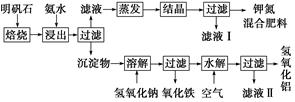
��������ͼʾ�����������գ�
��1������ʯ���պ���ϡ��ˮ����������500 mLϡ��ˮ(ÿ������39.20 g��)��ҪȡŨ��ˮ(ÿ������251.28 g��)__________mL���ù��Ϊ__________mL��Ͳ��ȡ��
��2����ˮ������õ���������ϵ�����ˣ���Һ�г�K����SO42-�⣬���д�����NH4+������NH4+�ķ�����_________________________________________________________________��
��3��д�����������������ʵĻ�ѧʽ___________________________________��
��4����Һ��ijɷ���ˮ��______________________��
��5��Ϊ�ⶨ��Ϸ���K2SO4��(NH4)2SO4�мصĺ��������������в��裺
�ٳ�ȡ�ص�������������ˮ����������__________��Һ��������ɫ������
��__________��__________��__________(������дʵ���������)��
����ȴ�����ء�
��6��������Ϊm g�����������ʵ���Ϊn mol����������K2SO4�����ʵ���Ϊ__________mol(�ú���m��n�Ĵ���ʽ��ʾ)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��������֮�������·�Ӧ��ϵ��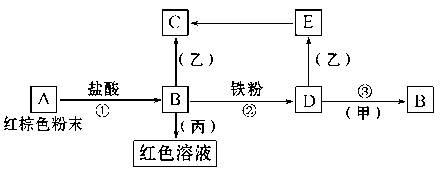
��֪��Eת����C�������ǣ���ɫ����Ѹ�ٱ�Ϊ����ɫ,����Ϊ���ɫ���ش�
��1��д���������ʵĻ�ѧʽ����________,��________��
��2��д��E��C��Ӧ�Ļ�ѧ����ʽ��___________________________��Ϊ�˻��E�������������Ƶ�D��Һ���ò���O2������ˮ���Ƶ�����Һ��Ӧ�Ʊ���
��3������Ӧ������������D��Һʱ����û������ ��
��4��д��Fe��H20��һ�������·�Ӧ�Ļ�ѧ����ʽ ��������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��ͼ�Ǵ�ͳ�Ĺ�ҵ�����������Ļ�������ͼ��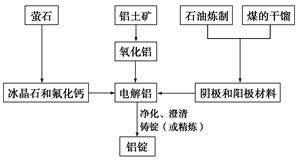
�������ͼ�ش��������⣺
��1����ҵұ���������õ������������������Ҫ�ɷ���________(�ѧʽ)��ú�ĸ������__________(����������)����������������������ϡ�
��2���������۵�ߴ�2050�棬��ҵ��Ϊ�˽����������ģ��ڽ�������ұ�������в�ȡ�Ĵ�ʩ��________________________________________________________________________��
��3����ұ�������У�����������Ҫ���ڵؽ��и�����ԭ���Ǹü����ϲ��ϱ����ģ��������������ԭ����__________________________________________________________��
��4����ҵ����ȡ����þ��ȡ�������MgCl2�ķ�������ⷴӦ����ʽΪ_________________��þ�������ǻ��ý�����Ϊʲô�ڵ��ұ�������У�һ�����Ȼ��һ���������________________________________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
(1)���û�ѧ��Ӧ�����Ʊ��������ʡ�ʵ������ͭ�Ʊ�NO2�����ӷ���ʽΪ_____________ ______��
��2����ҵ�ϣ���ͭ����Ҫ�ɷ�CuFeS2������ȡͭ����Ҫԭ�ϣ��ɲ��û�������������ͭ���ù��յ��м���̻ᷢ����Ӧ��Cu2S��2Cu2O===6Cu+SO2�����÷�Ӧ�л�ԭ��Ϊ_______ (�ѧʽ)��ÿ����1mol Cu����Ӧ��ת�Ƶ��ӵ����ʵ���Ϊ___________��
��ͭ��ұ��ͭ������¯������Fe2O3��FeO��SiO2��Al2O3�����Ʊ�Fe2O3������Ϊ��
����ϡ�����ȡ¯�������ˡ� ����Һ���������ټ������NaOH��Һ�����ˣ�������ϴ�ӡ�������յ�Fe2O3�� ��������Ϣ�ش��������⣺
a��ͨ�������ڣ�¯���е�Al2O3����� ��д���ӣ���
b��ѡ���ṩ���Լ������ʵ����֤¯���к���FeO��
�ṩ���Լ���ϡ���� ϡ���� KSCN��Һ ����KMnO4��Һ NaOH��Һ ��ˮ
��ѡ�Լ�Ϊ ��
֤��¯���к���FeO��ʵ������Ϊ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��1��ͭ����������ʹ�õĽ���֮һ��������ұ��ͭ��һ����Ӧ��
Cu2S+2Cu2O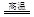 6Cu+SO2
6Cu+SO2
�÷�Ӧ�б�������Ԫ���� ������Ӧ��ת��1mol���ӣ��õ�Cu mol��
��2���������ַ����������Ʊ�CuSO4��
����һ��2Cu+O2 2CuO��CuO+H2SO4=CuSO4+H2O
2CuO��CuO+H2SO4=CuSO4+H2O
��������Cu+2H2SO4��Ũ�� CuSO4+SO2��+2H2O
CuSO4+SO2��+2H2O
�ټ���ij����������CuSO4����ѡ��һ�ַ�������˵�����ɣ�
��
����ͬѧ��Ϊ�����ɵ���������ͭ���ַ������ĵ�������ͬ����Ϊ��Ӧ����ͭת��ΪCuSO4������Ϊ����˵�� �����ȷ������ȷ������ԭ����
��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�ɻ�ͭ��(��Ҫ�ɷ���CuFeS2)���ƾ�ͭ�Ĺ�������ʾ��ͼ���£�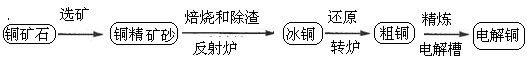
��1���ڷ���¯�У���ͭ����ɰ��ʯӢɰ��ϼ��ȵ�1000�����ң���ͭ���������Ӧ����Cu��Fe�ĵͼ�����Ҳ���Fe������ת��Ϊ�ͼ�������ù�����������Ҫ��Ӧ�Ļ�ѧ����ʽ�� �� ������¯������¯������Ҫ�ɷ��� ��
��2����ͭ(Cu2S��FeS�����ۺ϶���)��Cu��Ϊ20%��50%��ת¯�У�����ͭ���ۼ�(ʯӢɰ)��1200�����Ҵ���������д�������ͭ�е�Cu2S��������Cu2O�����ɵ�Cu2O��Cu2S��Ӧ�����ɺ�Cu��ԼΪ98.5%�Ĵ�ͭ���ù��̷�����Ӧ�Ļ�ѧ����ʽ�� �� ��
��3����ͭ�ĵ�⾫����ͼ��ʾ���ڴ�ͭ�ĵ������У���ͭ����ͼ�е缫 (��ͼ�е���ĸ)���ڵ缫d�Ϸ����ĵ缫��ӦʽΪ ������ͭ�л�����Au��Ag��Fe�������ڵ����еĴ�����ʽ��λ��Ϊ ��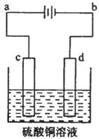
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��1����FeCl3��Һ�м���a g��ͭ�ۣ�����ʹ֮ȫ���ܽ⣬��Һ��һ���е���������_________�������е��������� ��������Ӧ�����ӷ���ʽΪ ���������м��� b g���ۣ���ַ�Ӧ����˵�����c g�����ܷ�����Ӧ�����ӷ���ʽΪ ������֪ a��b��c���� c��������_________��
��2������Na2SO3��Һ��ϡH2SO4���밴Ҫ������±���
| ���� | ���� | ������ |
| 1 | �üס�����֧�Թֱܷ�ȡ������Һ�������� | |
| 2 | �ý�ͷ�ι�����Թ�����εμ�BaCl2��Һ�������� | |
| 3 | �� | ������� ������ �� ������� ������ �� |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com