
| 8.10g |
| M |
| 0.18g |
| 18g/mol |
| 8.10g |
| M |
| 0.18g |
| 18g/mol |
| 4.05g |
| 810g/mol |
| 2.40g |
| 60g/mol |
| 4.05g |
| 810g/mol |
| 2.40g |
| 60g/mol |
| 4.05g |
| 810g/mol |
| 0.336L |
| 22.4L/mol |



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��������к͵ζ�ʵ���п��ô��м��������к͵ζ����� |
| B�����Թ��м���4mL 0.1mol?L-1K2Cr2O7��Һ���ٵμ�����1 mol?L-1NaOH��Һ����Һ��ɫ�ɻ�ɫ��Ϊ��ɫ |
| C�����Ʊ�����ؾ����ʵ���У����ȹ���ʱ���н���Һ��С�ձ����ȼ���2mL��ˮ���Է�����ʱ�ձ��е�����ؾ���������� |
| D������ʱ�¶ȼ�ˮ�������������ƿ֧�ܿڵ��·�λ�ã����ռ����߷е���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ����Һ�������壩X�����루��ͨ�룩��һ������Y��Һ�У������������������X���ʵ����Ĺ�ϵ��ͼ������ͼ�������һ�������ǣ�������
����Һ�������壩X�����루��ͨ�룩��һ������Y��Һ�У������������������X���ʵ����Ĺ�ϵ��ͼ������ͼ�������һ�������ǣ�������| A | B | C | D | |
| X | H2S | HCl | NH3 | NH3��H2O |
| Y | Na2SO3 | NaAlO2 | AlCl3 | AgNO3 |
| A��A | B��B | C��C | D��D |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ��O2��O3�� ��CH3CH��CH3��CH3��CH��CH3��3 ����ʯ��ʯī��
��O2��O3�� ��CH3CH��CH3��CH3��CH��CH3��3 ����ʯ��ʯī���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| �ɷ� | Fe2O3��KNO3��KOH��ϼ��ȹ��������Ϻ�ɫK2FeO4��KNO2�Ȳ��� |
| ʪ�� | ǿ���Խ����У�Fe��NO3��3��NaClO��Ӧ�����Ϻ�ɫNa2FeO4��Һ |
| ||
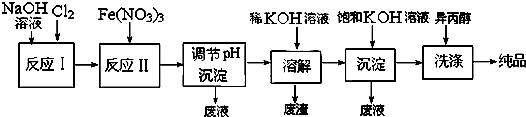
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
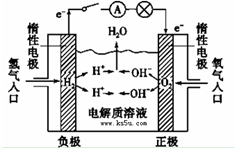 ��֪��1molH2��Ҫ����436kJ��������1molO2��Ҫ����496kJ�������γ�ˮ�����е�1molH-O�ܹ��ͷ�463kJ�����������������������ݼ��㷴Ӧ��
��֪��1molH2��Ҫ����436kJ��������1molO2��Ҫ����496kJ�������γ�ˮ�����е�1molH-O�ܹ��ͷ�463kJ�����������������������ݼ��㷴Ӧ���鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com