
НЋвЛЖЈСПДПОЛЕФАБЛљМзЫсяЇЙЬЬхжУгкЬижЦЕФУмБеецПеШнЦїжаЃЈМйЩшШнЦїЬхЛ§ВЛБфЃЌЙЬЬхЪдбљЬхЛ§КіТдВЛМЦЃЉЃЌдкКуЖЈЮТЖШЯТЪЙЦфДяЕНЗжНтЦНКтЃКNH2COONH4ЃЈsЃЉ  2NH3ЃЈgЃЉЃЋCO2ЃЈgЃЉ
2NH3ЃЈgЃЉЃЋCO2ЃЈgЃЉ
ЪЕбщВтЕУВЛЭЌЮТЖШЯТЕФЦНКтЪ§ОнСагкЯТБэЃК
ЮТЖШ/Ёц | 15.0 | 20.0 | 25.0 | 30.0 | 35.0 |
ЦНКтзмбЙ ЧП/kPa | 5.7 | 8.3 | 12.0 | 17.1 | 24.0 |
ЦНКтЦјЬхзмХЈЖШ/molЁЄ LЃ1 | 2.4ЁС 10Ѓ3 | 3.4ЁС 10Ѓ3 | 4.8ЁС 10Ѓ3 | 6.8ЁС 10Ѓ3 | 9.4ЁС 10Ѓ3 |
ЃЈ1ЃЉПЩвдХаЖЯИУЗжНтЗДгІвбОДяЕНЦНКтЕФЪЧ________ЁЃ
AЃЎ2vЃЈNH3ЃЉЃНvЃЈCO2ЃЉ
BЃЎУмБеШнЦїжазмбЙЧПВЛБф
CЃЎУмБеШнЦїжаЛьКЯЦјЬхЕФУмЖШВЛБф
DЃЎУмБеШнЦїжаАБЦјЕФЬхЛ§ЗжЪ§ВЛБф
ЃЈ2ЃЉИљОнБэжаЪ§ОнЃЌСаЪНМЦЫу25.0 ЁцЪБЕФЗжНтЗДгІЦНКтГЃЪ§ЃК_______________ЁЃ
ЃЈ3ЃЉШЁвЛЖЈСПЕФАБЛљМзЫсяЇЙЬЬхЗХдквЛИіДјЛюШћЕФУмБеецПеШнЦїжаЃЌдк25.0 ЁцЯТДяЕНЗжНтЦНКтЁЃШєдкКуЮТЯТбЙЫѕШнЦїЬхЛ§ЃЌАБЛљМзЫсяЇЙЬЬхЕФжЪСПНЋ________ЃЈЬюЁАдіМгЁБЁЂЁАМѕЩйЁБЛђЁАВЛБфЁБЃЉЁЃ
ЃЈ1ЃЉBC
ЃЈ2ЃЉKЃНc2ЃЈNH3ЃЉЁЄcЃЈCO2ЃЉЃН ЁЄ
ЁЄ ЃН
ЃН ЁСЃЈ4.8ЁС10Ѓ3ЃЉ3Ёж1.6ЁС10Ѓ8
ЁСЃЈ4.8ЁС10Ѓ3ЃЉ3Ёж1.6ЁС10Ѓ8
ЃЈ3ЃЉдіМг
ЁОНтЮіЁПЃЈ1ЃЉAЯюВЛФмБэЪОе§ЁЂФцЗДгІЫйТЪЯрЕШЃЛBЯюгЩгке§ЗДгІЗНЯђЦјЬхЗжзгЪ§діДѓЃЌдђУмБеШнЦїжабЙЧПВЛБфЃЌЗДгІДяЕНЦНКтЃЛCЯюКуШнЃЌШєЦНКтЗЂЩњвЦЖЏЃЌдђЛьКЯЦјЬхУмЖШЗЂЩњИФБфЃЛDЯюЗДгІЮяЪЧЙЬЬхЃЌNH3ЕФЬхЛ§ЗжЪ§ЪМжеЮЊ ЁЃ
ЁЃ
ЃЈ2ЃЉашНЋ25 ЁцЕФзмХЈЖШзЊЛЏЮЊNH3КЭCO2ЕФХЈЖШЃК
cЃЈNH3ЃЉЃН ЁС4.8ЁС10Ѓ3 molЁЄLЃ1ЃН3.2ЁС10Ѓ3 molЁЄLЃ1ЃЌcЃЈCO2ЃЉЃН
ЁС4.8ЁС10Ѓ3 molЁЄLЃ1ЃН3.2ЁС10Ѓ3 molЁЄLЃ1ЃЌcЃЈCO2ЃЉЃН ЁС4.8ЁС10Ѓ3 molЁЄLЃ1ЃН1.6ЁС10Ѓ3 molЁЄLЃ1ЃЌ
ЁС4.8ЁС10Ѓ3 molЁЄLЃ1ЃН1.6ЁС10Ѓ3 molЁЄLЃ1ЃЌ
KЃНЃЈ3.2ЁС10Ѓ3ЃЉ2ЁС1.6ЁС10Ѓ3Ёж1.6ЁС10Ѓ8ЁЃ
ЃЈ3ЃЉдіДѓбЙЧПЃЌЦНКтЯђФцЗДгІЗНЯђвЦЖЏЃЌЙЬЬхжЪСПдіДѓЁЃ



| ФъМЖ | ИпжаПЮГЬ | ФъМЖ | ГѕжаПЮГЬ |
| ИпвЛ | ИпвЛУтЗбПЮГЬЭЦМіЃЁ | ГѕвЛ | ГѕвЛУтЗбПЮГЬЭЦМіЃЁ |
| ИпЖў | ИпЖўУтЗбПЮГЬЭЦМіЃЁ | ГѕЖў | ГѕЖўУтЗбПЮГЬЭЦМіЃЁ |
| ИпШ§ | ИпШ§УтЗбПЮГЬЭЦМіЃЁ | ГѕШ§ | ГѕШ§УтЗбПЮГЬЭЦМіЃЁ |
ПЦФПЃКИпжаЛЏбЇ РДдДЃК2014ФъИпЖўШЫНЬЛЏбЇбЁаоЮх2-1-2жЌЗОЬўШВЬўжЌЗОЬўРДдДМАгІгУСЗЯАОэЃЈНтЮіАцЃЉ ЬтаЭЃКЬюПеЬт
вбжЊЯТСаЮяжЪЃК

ЧыАДвЊЧѓЬюПеЃК
(1)аДГіЂйЂмЕФУћГЦ________ЁЂ________ЃЛ
(2)аДГіЂкЁЂЂнЕФЗжзгЪН________ЁЂ________ЃЛ
(3)ЛЅЮЊЭЌЗжвьЙЙЬхЕФЪЧ________ЁЃ
(4)аДГіЂлгыЕШЮяжЪЕФСПЕФBr2ЗДгІЕФЛЏбЇЗНГЬЪНЃК_______________________________ЁЃ
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК2014ФъИпжаЛЏбЇЖўТжДДаТбЕСЗЩЯзЈЬт1ЮяжЪзщГЩЗжРраджЪМАЛЏбЇгУгяСЗЯАОэЃЈНтЮіАцЃЉ ЬтаЭЃКбЁдёЬт
ЖдH2OЕФЕчРыЦНКтВЛВњЩњгАЯьЕФЮЂСЃЪЧЃЈ ЃЉЁЃ
AЃЎ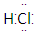 BЃЎ26M3ЃЋ C.
BЃЎ26M3ЃЋ C. DЃЎ
DЃЎ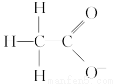
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК2014ФъИпжаЛЏбЇЖўТжДДаТбЕСЗЩЯ зЈЬт7ЕчНтжЪШмвКСЗЯАОэЃЈНтЮіАцЃЉ ЬтаЭЃКЬюПеЬт
ЛЗОГжаГЃМћЕФжиН№ЪєЮлШОЮягаЃКЙЏЁЂЧІЁЂУЬЁЂИѕЁЂягЁЃДІРэЙЄвЕЗЯЫЎжаКЌгаЕФCr2O72-КЭCrO42-ЃЌГЃгУЕФЗНЗЈгаСНжжЁЃ
ЗНЗЈ1ЁЁЛЙдГСЕэЗЈ
ИУЗЈЕФЙЄвеСїГЬЮЊ ЁЃ
ЁЃ
ЦфжаЕкЂйВНДцдкЦНКт2CrO42-ЃЈЛЦЩЋЃЉЃЋ2HЃЋ Cr2O72-ЃЈГШЩЋЃЉЃЋH2OЁЃ
Cr2O72-ЃЈГШЩЋЃЉЃЋH2OЁЃ
ЃЈ1ЃЉаДГіЕкЂйВНЗДгІЕФЦНКтГЃЪ§БэДяЪН_________________________________ЁЃ
ЃЈ2ЃЉЙигкЕкЂйВНЗДгІЃЌЯТСаЫЕЗЈе§ШЗЕФЪЧ________ЁЃ
AЃЎЭЈЙ§ВтЖЈШмвКЕФpHПЩвдХаЖЯЗДгІЪЧЗёвбДяЦНКтзДЬЌ
BЃЎИУЗДгІЮЊбѕЛЏЛЙдЗДгІ
CЃЎЧПЫсадЛЗОГЃЌШмвКЕФбеЩЋЮЊГШЩЋ
ЃЈ3ЃЉЕкЂкВНжаЃЌЛЙд0.1 mol Cr2O72-ЃЌашвЊ________molЕФFeSO4ЁЄ7H2OЁЃ
ЃЈ4ЃЉЕкЂлВНГ§ЩњГЩCrЃЈOHЃЉ3ЭтЃЌЛЙПЩФмЩњГЩЕФГСЕэЮЊ________ЁЃдкШмвКжаДцдквдЯТГСЕэШмНтЦНКтЃКCrЃЈOHЃЉ3ЃЈsЃЉ Cr3ЃЋЃЈaqЃЉЃЋ3OHЃЃЈaqЃЉЃЌГЃЮТЯТЃЌCrЃЈOHЃЉ3ЕФШмЖШЛ§KspЃН10Ѓ32ЃЌЕБcЃЈCr3ЃЋЃЉНЕжС10Ѓ5 molЁЄLЃ1ЪБЃЌШЯЮЊcЃЈCr3ЃЋЃЉвбОЭъШЋГСЕэЃЌЯжНЋЕкЂлВНШмвКЕФpHЕїжС4ЃЌЧыЭЈЙ§МЦЫуЫЕУїCr3ЃЋЪЧЗёГСЕэЭъШЋЃЈЧыаДГіМЦЫуЙ§ГЬЃЉЃК____________________________________________________________________________ЁЃ
Cr3ЃЋЃЈaqЃЉЃЋ3OHЃЃЈaqЃЉЃЌГЃЮТЯТЃЌCrЃЈOHЃЉ3ЕФШмЖШЛ§KspЃН10Ѓ32ЃЌЕБcЃЈCr3ЃЋЃЉНЕжС10Ѓ5 molЁЄLЃ1ЪБЃЌШЯЮЊcЃЈCr3ЃЋЃЉвбОЭъШЋГСЕэЃЌЯжНЋЕкЂлВНШмвКЕФpHЕїжС4ЃЌЧыЭЈЙ§МЦЫуЫЕУїCr3ЃЋЪЧЗёГСЕэЭъШЋЃЈЧыаДГіМЦЫуЙ§ГЬЃЉЃК____________________________________________________________________________ЁЃ
ЗНЗЈ2ЁЁЕчНтЗЈ
ЃЈ5ЃЉЪЕбщЪвРћгУШчЭМзАжУФЃФтЕчНтЗЈДІРэКЌCr2O72-ЕФЗЯЫЎЃЌЕчНтЪБбєМЋЗДгІЪНЮЊ________ЃЌвѕМЋЗДгІЪНЮЊ________ЃЌЕУЕНЕФН№ЪєбєРызгдквѕМЋЧјПЩГСЕэЭъШЋЃЌДгЫЎЕФЕчРыЦНКтНЧЖШНтЪЭЦфдвђЪЧ___________________________________________________________ЁЃ
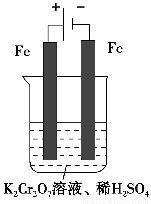
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК2014ФъИпжаЛЏбЇЖўТжДДаТбЕСЗЩЯ зЈЬт7ЕчНтжЪШмвКСЗЯАОэЃЈНтЮіАцЃЉ ЬтаЭЃКбЁдёЬт
ЯТСавКЬхОљДІгк25 ЁцЃЌгаЙиа№Ъіе§ШЗЕФЪЧ ЃЈЁЁЁЁЃЉЁЃ
AЃЎФГЮяжЪШмвКЕФpHЃО7ЃЌдђИУЮяжЪвЛЖЈЪЧМюЛђЧПМюШѕЫсбЮ
BЃЎpHЃН6.5ЕФХЃФЬжаcЃЈHЃЋЃЉЪЧpHЃН4.5ЕФH2SO4ШмвКжаcЃЈHЃЋЃЉЕФ100БЖ
CЃЎpHЃН3ЕФДзЫсгыpHЃН11ЕФNaOHШмвКЕШЬхЛ§ЛьКЯКѓШмвКжаЃКcЃЈCH3COOЃЃЉЃОcЃЈNaЃЋЃЉЃОcЃЈHЃЋЃЉЃОcЃЈOHЃЃЉ
DЃЎAgClдкЕШХЈЖШЕФCaCl2ШмвККЭNaClШмвКжаЕФШмНтЖШЯрЭЌ
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК2014ФъИпжаЛЏбЇЖўТжДДаТбЕСЗЩЯ зЈЬт6ЛЏбЇЗДгІЫйТЪКЭЛЏбЇЦНКтСЗЯАОэЃЈНтЮіАцЃЉ ЬтаЭЃКбЁдёЬт
дкKIШмвКжаДцдкЯТСаЦНКтЃКI2ЃЈaqЃЉЃЋIЃЃЈaqЃЉ=I3-ЃЈaqЃЉЁЃВтЕУВЛЭЌЮТЖШЯТИУЗДгІЕФЦНКтГЃЪ§KШчБэЫљЪОЃК
t/Ёц | 5 | 15 | 25 | 35 | 50 |
K | 1 100 | 841 | 689 | 533 | 409 |
ЯТСаЫЕЗЈе§ШЗЕФЪЧ ЃЈЁЁЁЁЃЉЁЃ
AЃЎЗДгІI2ЃЈaqЃЉЃЋIЃЃЈaqЃЉ  I3-ЃЈaqЃЉЕФІЄH>0
I3-ЃЈaqЃЉЕФІЄH>0
BЃЎЦфЫћЬѕМўВЛБфЃЌЩ§ИпЮТЖШЃЌШмвКжаcЃЈI3-ЃЉМѕаЁ
CЃЎИУЗДгІЕФЦНКтГЃЪ§БэДяЪНЮЊKЃН
DЃЎ25 ЁцЪБЃЌЯђШмвКжаМгШыЩйСПKIЙЬЬхЃЌЦНКтГЃЪ§KаЁгк689
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК2014ФъИпжаЛЏбЇЖўТжДДаТбЕСЗЩЯ зЈЬт5ЛЏбЇЗДгІгыФмСПБфЛЏСЗЯАОэЃЈНтЮіАцЃЉ ЬтаЭЃКЬюПеЬт
ЃЈ1ЃЉгЩСзЛвЪЏ[жївЊГЩЗжCa5ЃЈPO4ЃЉ3F]дкИпЮТЯТжЦБИЛЦСзЃЈP4ЃЉЕФШШЛЏбЇЗНГЬЪНЮЊЃК4Ca5ЃЈPO4ЃЉ3FЃЈsЃЉЃЋ21SiO2ЃЈsЃЉЃЋ30CЃЈsЃЉ=3P4ЃЈgЃЉЃЋ20CaSiO3ЃЈsЃЉЃЋ30COЃЈgЃЉЃЋSiF4ЃЈgЃЉЁЁІЄH
ЂйЩЯЪіЗДгІжаЃЌИБВњЮяПѓдќПЩгУРД________ЁЃ
ЂквбжЊЯрЭЌЬѕМўЯТЃК
4Ca5ЃЈPO4ЃЉ3FЃЈsЃЉЃЋ3SiO2ЃЈsЃЉ=6Ca3ЃЈPO4ЃЉ2ЃЈsЃЉЃЋ2CaSiO3ЃЈsЃЉЃЋSiF4ЃЈgЃЉЁЁІЄH1
2Ca3ЃЈPO4ЃЉ2ЃЈsЃЉЃЋ10CЃЈsЃЉ=P4ЃЈgЃЉЃЋ6CaOЃЈsЃЉЃЋ10COЃЈgЃЉЁЁІЄH2
SiO2ЃЈsЃЉЃЋCaOЃЈsЃЉ=CaSiO3ЃЈsЃЉЁЁІЄH3
гУІЄH1ЁЂІЄH2КЭІЄH3БэЪОІЄHЃЌІЄHЃН____________ЁЃ
ЃЈ2ЃЉЃЈНЫеЃЉгУH2O2КЭH2SO4ЕФЛьКЯШмвКПЩШмГігЁЫЂЕчТЗАхН№ЪєЗлФЉжаЕФЭЁЃвбжЊЃК
ЂйCuЃЈsЃЉЃЋ2HЃЋЃЈaqЃЉ=Cu2ЃЋЃЈaqЃЉЃЋH2ЃЈgЃЉІЄH1ЃНЃЋ64.39 kJЁЄmolЃ1
Ђк2H2O2ЃЈlЃЉ=2H2OЃЈlЃЉЃЋO2ЃЈgЃЉІЄH2ЃНЃ196.46 kJЁЄmolЃ1
ЂлH2ЃЈgЃЉЃЋ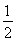 O2ЃЈgЃЉ=H2OЃЈlЃЉІЄH3ЃНЃ285.84 kJЁЄmolЃ1
O2ЃЈgЃЉ=H2OЃЈlЃЉІЄH3ЃНЃ285.84 kJЁЄmolЃ1
дкH2SO4ШмвКжаЃЌCuгыH2O2ЗДгІЩњГЩCu2ЃЋКЭH2OЕФШШЛЏбЇЗНГЬЪНЮЊ_________________________________________________________________ЁЃ
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК2014ФъИпжаЛЏбЇЖўТжДДаТбЕСЗЩЯ зЈЬт4ЮяжЪНсЙЙгыдЊЫижмЦкТЩСЗЯАОэЃЈНтЮіАцЃЉ ЬтаЭЃКбЁдёЬт
ЯТСаЙигкжИЖЈСЃзгЙЙГЩЕФа№ЪіжаЃЌВЛе§ШЗЕФЪЧ ЃЈЁЁЁЁЃЉЁЃ
AЃЎ37Clгы39KОпгаЯрЭЌЕФжазгЪ§
BЃЎЕк114КХдЊЫиЕФвЛжжКЫЫи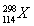 гы
гы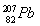 ОпгаЯрЭЌЕФзюЭтВуЕчзгЪ§
ОпгаЯрЭЌЕФзюЭтВуЕчзгЪ§
CЃЎH3OЃЋгыOHЃОпгаЯрЭЌЕФжЪзгЪ§КЭЕчзгЪ§
DЃЎO22-гыS2ЃОпгаЯрЭЌЕФжЪзгЪ§КЭЕчзгЪ§
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК2014ФъИпжаЛЏбЇЖўТжДДаТбЕСЗЩЯ зЈЬт13ЛЏбЇЪЕбщзлКЯгІгУСЗЯАОэЃЈНтЮіАцЃЉ ЬтаЭЃКЬюПеЬт
ЖўбѕЛЏТШЃЈClO2ЃЉЪЧФПЧАЙњМЪЩЯЙЋШЯЕФЕкЫФДњИпаЇЁЂЮоЖОЕФЯћЖОМСЃЌЪЧвЛжжЛЦТЬЩЋЕФЦјЬхЃЌвзШмгкЫЎЁЃ
Ђё.ЃЈ1ЃЉClO2ПЩгЩKClO3дкH2SO4ДцдкЕФЬѕМўЯТгыNa2SO3ЗДгІжЦЕУЁЃдђИУЗДгІЕФбѕЛЏВњЮягыЛЙдВњЮяЕФЮяжЪЕФСПжЎБШЪЧ________ЁЃ
Ђђ.ЪЕбщЪввВПЩгУNH4ClЁЂбЮЫсЁЂNaClO2ЃЈбЧТШЫсФЦЃЉЮЊдСЯжЦБИClO2ЃЌЦфСїГЬШчЯТЃК

ЃЈ2ЃЉаДГіЕчНтЪБЗЂЩњЗДгІЕФЛЏбЇЗНГЬЪНЃК________________________________ЁЃ
ЃЈ3ЃЉГ§ШЅClO2жаЕФNH3ПЩбЁгУЕФЪдМСЪЧ________ЁЃЃЈЬюађКХЃЉ
AЃЎБЅКЭЪГбЮЫЎ BЃЎМюЪЏЛв
CЃЎХЈСђЫс DЃЎЫЎ
ЃЈ4ЃЉВтЖЈClO2ЃЈШчЭМЃЉЕФЙ§ГЬШчЯТЃКдкзЖаЮЦПжаМгШызуСПЕФЕтЛЏМиЃЌгУ100 mLЫЎШмНтКѓЃЌдйМг3 mLСђЫсШмвКЃЛдкВЃСЇвКЗтЙмжаМгШыЫЎЃЛНЋЩњГЩЕФClO2ЦјЬхЭЈЙ§ЕМЙмдкзЖаЮЦПжаБЛЮќЪеЃЛНЋВЃСЇЗтЙмжаЕФЫЎЗтвКЕЙШызЖаЮЦПжаЃЌМгШыМИЕЮЕэЗлШмвКЃЌгУc molЁЄLЃ1СђДњСђЫсФЦБъзМШмвКЕЮЖЈЃЈI2ЃЋ2S2O32-=2IЃЃЋS4O62-ЃЉЃЌЙВгУШЅV mLСђДњСђЫсФЦШмвКЁЃ

ЂйзАжУжаВЃСЇвКЗтЙмЕФзїгУЪЧ______________________________________ЁЃ
ЂкЧыаДГіЩЯЪіЖўбѕЛЏТШЦјЬхгыЕтЛЏМиШмвКЗДгІЕФРызгЗНГЬЪН__________________________ЁЃ
ЂлЕЮЖЈжеЕуЕФЯжЯѓЪЧ_______________________________________________ЁЃ
ЂмВтЕУЭЈШыClO2ЕФжЪСПmЃЈClO2ЃЉЃН________ЁЃЃЈгУКЌcЁЂVЕФДњЪ§ЪНБэЪОЃЉ
ЃЈ5ЃЉгУClO2ДІРэЙ§ЕФвћгУЫЎЃЈpHЮЊ5.5ЁЋ6.5ЃЉГЃКЌгавЛЖЈСПЖдШЫЬхВЛРћЕФбЧТШЫсИљРызгClO2-ЁЃ2001ФъЮвЙњЮРЩњВПЙцЖЈЃЌвћгУЫЎЕФClO2-КЌСПгІВЛГЌЙ§0.2 mgЁЄLЃ1ЁЃШєвћгУЫЎжаClO2-ЕФКЌСПГЌБъЃЌПЩЯђЦфжаМгШыЪЪСПЕФФГЛЙдМСЃЌИУЗДгІЕФбѕЛЏВњЮяЪЧ________ЃЈЬюЛЏбЇЪНЃЉЃЌЦфЗЂЩњЗДгІЕФРызгЗНГЬЪНЮЊ_________________________________________________________________ЁЃ
ВщПДД№АИКЭНтЮі>>
ЙњМЪбЇаЃгХбЁ - СЗЯАВсСаБэ - ЪдЬтСаБэ
КўББЪЁЛЅСЊЭјЮЅЗЈКЭВЛСМаХЯЂОйБЈЦНЬЈ | ЭјЩЯгаКІаХЯЂОйБЈзЈЧј | ЕчаХеЉЦОйБЈзЈЧј | ЩцРњЪЗащЮожївхгаКІаХЯЂОйБЈзЈЧј | ЩцЦѓЧжШЈОйБЈзЈЧј
ЮЅЗЈКЭВЛСМаХЯЂОйБЈЕчЛАЃК027-86699610 ОйБЈгЪЯфЃК58377363@163.com