
��32.64gͭ��140mLһ��Ũ�ȵ����ᷴӦ��ͭ��ȫ�ܽ������NO��NO2��������ڱ�״���µ����Ϊ11.2L����ش�
��1��NO�����Ϊ L��NO2�����Ϊ L��
��2��������������ȫ���ͷź�����Һ����V mL amol��L��1��NaOH��Һ��ǡ��ʹ��Һ�е�Cu2��ȫ��ת���ɳ�������ԭ������Һ��Ũ��Ϊ mol/L��
��3����ʹͭ�����ᷴӦ���ɵ�������NaOH��Һ��ȫ��ת��ΪNaNO3��������Ҫ30%��˫��ˮ g��
��1��5.824 L 5.376 L ��2��(10-3 va +0.5)/0.14 ��3��57.8 g
���������������1���跴Ӧ�в���NO��NO2������ֱ�Ϊx��y(�ݵ�ʧ�����غ���)
x+y=11.2
(x/22.4)��3+(y/22.4) ��1=(32.64/64)��2
���x="5.824" L y="5.376" L
��2�����ݷ�Ӧ�����Һ��ֻ��һ������NaNO3 ,��ԭ��������ʵ���Ũ��ΪC
���ɵ�Ԫ���غ㣩�� 0.14��C= 10-3 ��v��a + 11.2/22.4
��ԭ�����Ũ��Ϊ�� C= (10-3 va +0.5)/0.14
��3���ӷ�Ӧ��ʼ̬������״̬��,ͭ�ڷ�Ӧ��ʧȥ����,˫��ˮ�ڷ�Ӧ�еõ�����,��Ҫ30%��˫��ˮ������Ϊm ��(�ݵ�ʧ�����غ���)��(32.64/64)��2=��(30%��m)/34����2 ���:m="57.8" g
���㣺������ԭ��Ӧ���ɣ�������ԭ��Ӧ����ʽ�ļ��㡣



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
������Դ����Ľ�һ��ͻ���������Ȼ�ѧѭ��������о��ܵ��������ҵ�������������о����֣�����������������(MnFe2O4)Ҳ���������Ȼ�ѧѭ���ֽ�ˮ���⣬MnFe2O4���Ʊ��������£�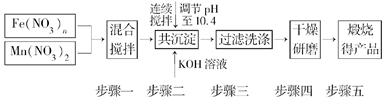
(1)ԭ��Fe(NO3)n��n��________��Ͷ��ԭ��Fe(NO3)n��Mn(NO3)2�����ʵ���֮��ӦΪ________��
(2)������С��������衱��Ŀ����__________________________________________
��������ϴ�Ӹɾ��ı���________________________________________________
(3)����MnFe2O4�Ȼ�ѧѭ������ķ�Ӧ�ɱ�ʾΪ��
MnFe2O4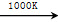 MnFe2O4��x��O2����
MnFe2O4��x��O2����
MnFe2O4��x��xH2O MnFe2O4��xH2��
MnFe2O4��xH2��
�������������������Ӧ���ش��������⣺
����MnFe2O4��x��x��0.8����MnFe2O4��x��Fe2��ռȫ����Ԫ�صİٷ���Ϊ________��
�ڸ��Ȼ�ѧѭ�����ⷨ���ŵ���_____________________��________________________ (�����㼴��)��
���Ȼ�ѧѭ�����������в���֮������һ���Ľ����о�������___________________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��ҵ�����ý�̿��ʯ��Ҥ��ȼ�շ��ȣ�ʹʯ��ʯ�ֽ�����CO2����Ҫ��Ӧ���£�
C+O2��CO2 �٣� CaCO3��CO2��+CaO ��
��1����̼���95%��ʯ��ʯ2.0 t������ȫ�ֽ⣨�����ʲ��ֽ⣩���ɵñ�״����CO2�����Ϊ_________________m3��
��2��������CaCO3�ͽ�̿���٢���ȫ��Ӧ����Ҥ�������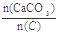 =2.2ʱ��Ҥ����CO2������������Ϊ���٣��������ֻ��N2��O2���������Ϊ4��1����ͬ��
=2.2ʱ��Ҥ����CO2������������Ϊ���٣��������ֻ��N2��O2���������Ϊ4��1����ͬ��
��3��ij��Ҥ���ɷ����£�O2 0.2%��CO 0.2%��CO2 41.6%������ΪN2����˴�Ҥ������� Ϊ��ֵ��
Ϊ��ֵ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��11.2g��Ͷ��200mLijŨ�ȵ������У���������ǡ����ȫ��Ӧ����
��1������������HCl�����ʵ���Ũ��
��2����Ӧ�����ɵ�H2�ڱ�״���µ����
��3���ڷ�Ӧ�����Һ��ͨ��Cl2��д����������Ӧ�Ļ�ѧ����ʽ����˫���ű������ת�Ƶķ������Ŀ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��1����ag�Ȼ�������1.8Lˮ�У�ǡ��ʹ����������ˮ������֮��Ϊ1:100����aֵΪ ��
��2���ڷ�Ӧ2A+B=3C+2D�У���֪3.4gA��3.2gB��ȫ��Ӧ������4.8gC����֪��D��ʽ��Ϊ18����B��ʽ����
��3��25.4g ij���۽����Ȼ���(ACl2)�к���0.4mol Cl������ACl2��Ħ�������� ��A�����ԭ�������� ��ACl2�Ļ�ѧʽ�� ��
��4�� ij�������Na2SO4��Al2��SO4��3��ɣ���֪Na��Al��Ԫ�ص�����֮��Ϊ23: 9����Na2SO4��Al2��SO4��3���ʵ���֮��Ϊ ����1.00mol SO42�C�ĸû���������Ϊ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
ʵ�������Ȼ��ƹ�������1��00 mol/L��NaCl��Һ0��5 L���ش��������⣺
��1����д����ʵ���ʵ�鲽�裺
�� ���� ���� ���� ���� ���� ��
��2����������Ϊ��������ƽ������Ҫ��Щʵ������������ɸ�ʵ�飬��д���� ��
��3���Է������в�����������Һ��Ũ���к�Ӱ�켰��ɸ�Ӱ���ԭ��
���ݺӸǵ�תҡ�Ⱥ���Һ����ڿ̶��ߣ��ֵμ�����ˮ���̶ȡ���������ҺŨ�ȵ�Ӱ�죺 ��ԭ���ǣ� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
ʵ����Ҫ0.10mol/LNaOH��Һ470mL��������Һ����������ش��������⣺
��1��ʵ���г���������ƽ���ձ�������������Ͳ��ҩ�����Ҫ�����������У� ��
��2�����ݼ����֪������NaOH������Ϊ g��
��3������ʱ��������ƿ����Һ�İ�Һ��������̶������У��Ǻ�ƿ�������һ�������� ��
��4������ʱ,�������ˮ�����̶��ߣ������ȡ�Ĵ�ʩ�ǣ� ��
��5�����в���������Ũ���к�Ӱ�죨��д��ĸ��
ƫ�͵��� ����Ӱ����� ��
| A������������������룻 |
| B����NaOH����ֽ���ϳ����� |
| C��NaOH���ձ����ܽ��δ��ȴ������ת�Ƶ�����ƿ�У� |
| D������ʱ���ӿ̶��� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
Ҫȷ���ջ�ѧ���P���ü�����������Ҫ��ش��������⣺
��1��NA��ʾ�����ӵ�������28g��ϩ�ͻ����飨C4H8���Ļ�������к���____NA��̼ԭ�ӣ���������ΪNA����NO2��CO2������庬______ NA����ԭ������1mol37Cl�У�����������������_______ NA����1L 1mol/LFe2(SO4)3��Һ�к�_____NA��SO42�����ӡ�
��2��ͼ��A��B�ֱ���ij���Ľṹʾ��ͼ���ش��������⣺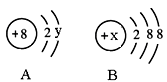
����A��ʾijԪ�ص�ԭ�ӣ���y�� ��
����B��ʾijϡ������Ԫ�ص�ԭ�ӣ����Ԫ�صĵ��ʵĻ�ѧʽΪ ����B�������ӵĽṹʾ��ͼ����x��ȡֵ��Χ��________________��
��3��RxO42����R�Ļ��ϼ�Ϊ___________(�ú�x ��ʽ�ӱ�ʾ)����0.3 mol RxO42����ȫ��Ӧ������RO2ʱ��ת��0.6 mol���ӣ���x��__________��
��4����7.8 gþ���Ͻ���100mL ϡ����ǡ����ȫ��Ӧ������Ӧ�����Һ�������ɣ��õ���ˮ������46.2 g����ԭ��������ʵ���Ũ��Ϊ______________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�������
��һ���������Ļ����Һ��ȡ��20mL����������BaCl2��Һ�������ˡ�ϴ�ӡ���ɺ�ó���9.32g����Һ��4.0mol/L������������Һ35mLǡ����ȫ�к͡�����
��1��ԭ�����Һ���������������ʵ���Ũ�ȡ�
��2����ȡ10mLԭ��Һ������0.96gͭ�۹��ȣ�����һ�����������Ϊ���٣�
��3����ȡ10mLԭ��Һ������1.92gͭ�۹���,�����ٻ�Ҫ��Ӧ�����Һ�м�����ٺ���1mol/L������ܽ�ͭ��ǡ����ȫ�ܽ⣿
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com