
��Դ�Ŀ����������������Ŀɳ�����չϢϢ��ء�
��.��֪��Fe2O3��s����3C��s��=2Fe��s����3CO��g��
��H1��a kJ��mol��1
CO��g����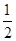 O2��g��=CO2��g������H2��b kJ��mol��1
O2��g��=CO2��g������H2��b kJ��mol��1
4Fe��s����3O2��g��=2Fe2O3��s������H3��c kJ��mol��1
��C��ȼ���Ȧ�H��________ kJ��mol��1��
��.��1������ԭ��صĹ���ԭ�������л�ѧ��Ӧ�������Ͽ�����Ƴ�ԭ��ص���________������ţ���
A��C��s����CO2��g��=2CO��g��
B��NaOH��aq����HCl��aq��=NaCl��aq����H2O��l��
C��2H2O��l��=2H2��g����O2��g��
D��2CO��g����O2��g��=2CO2��g��
�������ڵ�K2CO3��CO2Ϊ��Ӧ�Ļ�����������ѡ��Ӧ��Ƴ�һ��ԭ��أ���д����ԭ��صĸ�����Ӧ��____________________________________________________________��
��2��ijʵ��С��ģ�ҵ�ϳɰ���ӦN2��g����3H2��g�� 2NH3��g������H����92.4 kJ��mol��1����ʼ���ǽ�N2��H2�������20 mol�������1��1������5 L�ϳ����У���ӦǰѹǿΪp0����Ӧ������ѹǿ��p��ʾ����Ӧ������
2NH3��g������H����92.4 kJ��mol��1����ʼ���ǽ�N2��H2�������20 mol�������1��1������5 L�ϳ����У���ӦǰѹǿΪp0����Ӧ������ѹǿ��p��ʾ����Ӧ������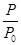 ��ʱ��t�Ĺ�ϵ��ͼ��ʾ��
��ʱ��t�Ĺ�ϵ��ͼ��ʾ��
��ش��������⣺
�ٷ�Ӧ��ƽ��ı�־�ǣ�����ĸ���ţ�________��
A��ѹǿ���ֲ���
B�������ܶȱ��ֲ���
C��NH3������������N2���������ʵ�2��
��0��2 min�ڣ���c��N2���仯��ʾ��ƽ����Ӧ����Ϊ________��
�������N2��ת���ʣ��ɲ�ȡ�Ĵ�ʩ��________��
A������ϵ�а������1��1�ٳ���N2��H2
B�������NH3
C�������¶�
D�����뺤��ʹѹǿ����
E������һ������N2
��3��25 ��ʱ��BaCO3��BaSO4���ܶȻ������ֱ���8��10��9��1��10��10��ij����BaCO3����������Һ�У�c��CO32-����0.2 mol��L��1���������������Na2SO4��Һ����Ҫ����BaSO4����������Na2SO4��Һ�����ʵ���Ũ����С��________ mol��L��1��
 ��ͼͼ�麮����ҵ������ҵ���ִ�ѧ������ϵ�д�
��ͼͼ�麮����ҵ������ҵ���ִ�ѧ������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��Ԫ�صĻ���������࣬����Ҳ������ͬ��
��1��NO2�н�ǿ�������ԣ��ܽ�SO2��������SO3����������ԭΪNO����֪��������Ӧ�����������仯��ͼ��ʾ��
��NO2����SO2���Ȼ�ѧ����ʽΪ_________________________________��
��2����2L�ܱ������з���1mol��������һ���¶Ƚ������·�Ӧ��
2NH3(g) N2��g��+3H2��g������Ӧʱ��(t)��������������ѹǿ(p)�����ݼ��±�
N2��g��+3H2��g������Ӧʱ��(t)��������������ѹǿ(p)�����ݼ��±�
| ʱ��t/min | 0 | 1 | 2 | 3 | 4 | 5 |
| ��ѹǿp 100 kPa | 5 | 5.6 | 6.4 | 6.8 | 7 | 7 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
������Ϊһ������Դ�ڻ�ѧ����Ӧ�ù㷺����ش��������⡣
��1����¯ұ�������У������ڴ���Ӧ���в���ˮú����CO��H2����ԭ���������йط�ӦΪ��CH4��g����CO2��g��=2CO��g����2H2��g������H��260 kJ��mol��1
��֪��2CO��g����O2��g��=2CO2��g����H����566 kJ��mol��1��
��CH4��O2��Ӧ����CO��H2���Ȼ�ѧ����ʽΪ____________________________________��
��2������ͼ��ʾ��װ�â�Ϊ����ȼ�ϵ�أ��������ҺΪKOH��Һ����ͨ��װ�â�ʵ�������϶�ͭ��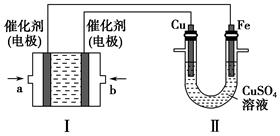
��a��Ӧͨ��________���CH4����O2������b���缫�Ϸ����ĵ缫��Ӧʽ��_________________________________________________________________��
�ڵ�ƽ�����װ�â�����Һ��pH________����д�������С�����䡱����ͬ����װ�â���Cu2�������ʵ���Ũ��________��
�۵�ƽ�����װ�â���Һ�е������ӳ���OH���������________������ˮ�⣩��
���ڴ˹���������ȫ��Ӧ��װ�â������������仯12.8 g����װ�â������������ļ���________L����״���£���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
���º���������,����Է�������ת��,�䷴Ӧ���̺�������ϵ��ͼ1��ʾ����֪:2SO2(g)+O2(g) 2SO3(g)��
2SO3(g)��
��H="-196.6" kJ/mol��
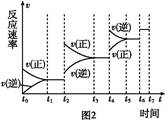
��ش���������:
(1)д���ܱ�ʾ���ȼ���ȵ��Ȼ�ѧ����ʽ: ��
(2)��H2=����������������������
(3)���º���ʱ,1 mol SO2��2 mol O2��ַ�Ӧ,�ų���������ֵ�ȨO��H2�O��������(�����С������ȡ�)��
(4)�����еĻ������ͨ��������NaOH��Һ������NaOH�����ʵ���Ϊ��������,����Һ�з�����������ԭ��Ӧ,��ù��̵����ӷ���ʽΪ�� ��
(5)����������,���д�ʩ����ʹn(SO3)/ n(SO2)�����������������
a.�����¶�
b.����He��
c.�ٳ���1 mol SO2(g)��1 mol O2(g)
d.ʹ�ô���
(6)ijSO2(g)��O2 (g)��ϵ,ʱ��t1�ﵽƽ���,�ı�ijһ�������,��Ӧ����v��ʱ��t�Ĺ�ϵ��ͼ2��ʾ,�����ı�SO2(g)��O2 (g)����,��ͼ��t4ʱ����ƽ���ƶ���������������������;ͼ�б�ʾƽ��������SO3�ĺ�����ߵ�һ��ʱ��������������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
CH4��H2��C�������ʵ���Դ���ʣ�����ȼ�յ��Ȼ�ѧ����ʽΪ��
��CH4(g)��2O2(g)=CO2(g)��2H2O(l)����H����890.3 kJ��mol��1��
��2H2(g)��O2(g)=2H2O(l)����H����571.6 kJ��mol��1��
��C(s)��O2(g)=CO2(g)����H����393.5 kJ��mol��1��
(1)����д���һ�ּ���ϸ������������øʹ������O2���ò���������������ϸ��ʹ1 mol��������CO2������Һ̬ˮ���ų�������________(���������������)890.3 kJ��
(2)������CO2�����ںϳɺϳ���(��Ҫ�ɷ���һ����̼������)��CH4��CO2=2CO��2H2��1 g CH4��ȫ��Ӧ���ͷ�15.46 kJ����������
����ͼ�ܱ�ʾ�÷�Ӧ�����������仯����________(����ĸ)��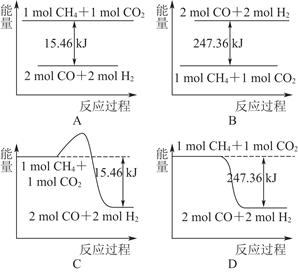
���������ʵ�����Ϊ1 mol��CH4��CO2����ij�����ܱ������У���ϵ�ų�����������ʱ��ı仯��ͼ��ʾ����CH4��ת����Ϊ________��
(3)C(s)��H2(g)����Ӧ������C(s)��2H2(g)=CH4(g)�ķ�Ӧ����ֱ�Ӳ�������ͨ��������Ӧ�������C(s)��2H2(g)=CH4(g)�ķ�Ӧ�Ȧ�H��________��
(4)Ŀǰ���������������ʵ��о���ȼ���о����ص㣬���й��������������ʵ��о������п��е���________(����ĸ)��
| A��Ѱ�����ʴ�����ʹCO2��H2O��Ӧ����CH4��O2�����ų����� |
| B��Ѱ�����ʴ������ڳ��³�ѹ��ʹCO2�ֽ�����̼��O2 |
| C��Ѱ�����ʴ���������̫����ʹ�����е�CO2�뺣���ɵ�CH4�ϳɺϳ���(CO��H2) |
| D������̬̼�ϳ�ΪC60����C60��Ϊȼ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��Դ�Ŀ����������������Ŀɳ�����չϢϢ��ء�
��.��֪��Fe2O3��s����3C��s��=2Fe��s����3CO��g��
��H1��a kJ��mol��1
CO��g����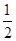 O2��g��=CO2��g���� ��H2��b kJ��mol��1
O2��g��=CO2��g���� ��H2��b kJ��mol��1
4Fe��s����3O2��g��=2Fe2O3��s���� ��H3��c kJ��mol��1
��C��ȼ���Ȧ�H��________kJ��mol��1��
��.��1������ԭ��صĹ���ԭ�������л�ѧ��Ӧ�������Ͽ�����Ƴ�ԭ��ص���________������ţ���
A��C��s����CO2��g��=2CO��g��
B��NaOH��aq����HCl��aq��=NaCl��aq����H2O��l��
C��2H2O��l��=2H2��g����O2��g��
D��2CO��g����O2��g��=2CO2��g��
�������ڵ�K2CO3��CO2Ϊ��Ӧ�Ļ�����������ѡ��Ӧ��Ƴ�һ��ԭ��أ���д����ԭ��صĸ�����Ӧ��_____________________________________��
��2��ijʵ��С��ģ�ҵ�ϳɰ���ӦN2��g����3H2��g�� 2NH3��g������H����92.4 kJ��mol��1����ʼ���ǽ�N2��H2�������20 mol�������1��1������5 L�ϳ����У���ӦǰѹǿΪP0����Ӧ������ѹǿ��P��ʾ����Ӧ������
2NH3��g������H����92.4 kJ��mol��1����ʼ���ǽ�N2��H2�������20 mol�������1��1������5 L�ϳ����У���ӦǰѹǿΪP0����Ӧ������ѹǿ��P��ʾ����Ӧ������ ��ʱ��t�Ĺ�ϵ��ͼ��ʾ��
��ʱ��t�Ĺ�ϵ��ͼ��ʾ��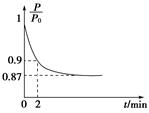
��ش��������⣺
�ٷ�Ӧ��ƽ��ı�־��__________________________������ĸ���ţ���ͬ����
A��ѹǿ���ֲ���
B�������ܶȱ��ֲ���
C��NH3������������N2���������ʵ�2��
��0��2 min�ڣ���c��N2���仯��ʾ��ƽ����Ӧ����Ϊ________________��
�������N2��ת���ʣ��ɲ�ȡ�Ĵ�ʩ��_____________________________��
A������ϵ�а������1��1�ٳ���N2��H2
B�������NH3
C�������¶�
D�����뺤��ʹѹǿ����
E������һ������N2
��3��25��ʱ��BaCO3��BaSO4���ܶȻ������ֱ���8��10��9��1��10��10��ij����BaCO3����������Һ�У�c��CO32-����0.2 mol��L��1���������������Na2SO4��Һ����Ҫ����BaSO4����������Na2SO4��Һ�����ʵ���Ũ����С��________mol��L��1��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��1����֪�� C(s)+O2(g)=CO2(g) ��H1����393.5 kJ/mol
C(s)+H2O(g)=CO(g)+H2(g) ��H2����131.3 kJ/mol
��ӦCO(g)+H2(g) +O2(g)= H2O(g)+CO2(g)����H= ____ ___kJ/mol��
��2����һ���ݵ��ܱ������У���CO��H2�ϳɼ״���CO(g)+2H2(g) CH3OH(g) ��H
CH3OH(g) ��H
���������β���˵���÷�Ӧ�Ѵﵽƽ��״̬����_______������ţ���
A��ÿ����1 mol CO��ͬʱ����2molH2
B��������������ʵ�������
C������CH3OH������������CO���������
D��CH3OH��CO��H2��Ũ�ȶ����ٷ����仯
��CO��ƽ��ת���ʣ��������¶ȡ�ѹǿ�Ĺ�ϵ��ͼ��ʾ��A��B�����ƽ�ⳣ��K(A)_______K(B)�����������=������,��ͬ������ͼ�жϦ�H _____0��
��ij�¶��£���2.0 mol CO��6.0 molH2����2 L���ܱ������У���ַ�Ӧ�ﵽƽ��ʱ���c(CO)="0.25" mol/L����CO��ת����= �����¶��µ�ƽ�ⳣ��K= ��������λ��Ч���֣���
��3�������¶�650���������ȼ�ϵ�أ���ú̿����CO��H2����������Ӧ�������CO2�Ļ������Ϊ������Ӧ����������缫����һ��������Li2CO3��Na2CO3���۵�����������ʡ������ĵ缫��ӦʽΪ��CO+H2��4e-+2CO32-=3CO2+H2O����õ�ص�������ӦʽΪ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
(1)(�㶫)����ʯ[��Ҫ�ɷ�Ca5(PO4)3F]�ڸ������Ʊ�����(P4)���Ȼ�ѧ����ʽΪ��4Ca5(PO4)3F(s)��21SiO2(s)��30C(s)===3P4(g)��20CaSiO3(s)��30CO(g)��SiF4(g)����H
��������Ӧ�У����������������________��
����֪��ͬ�����£�
4Ca5(PO4)3F(s)��3SiO2(s)===6Ca3(PO4)2(s)��2CaSiO3(s)��SiF4(g)����H1
2Ca3(PO4)2(s)��10C(s)===P4(g)��6CaO(s)��10CO(g)����H2
SiO2(s)��CaO(s)===CaSiO3(s)����H3
�æ�H1����H2�ͦ�H3��ʾ��H����H��____________��
(2)(����)��H2O2��H2SO4�Ļ����Һ���ܳ�ӡˢ��·�������ĩ�е�ͭ����֪��
��Cu(s)��2H��(aq)===Cu2��(aq)��H2(g) ��H1����64.39 kJ��mol��1
��2H2O2(l)===2H2O(l)��O2(g) ��H2����196.46 kJ��mol��1
��H2(g)��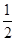 O2(g)===H2O(l) ��H3����285.84 kJ��mol��1
O2(g)===H2O(l) ��H3����285.84 kJ��mol��1
��H2SO4��Һ�У�Cu��H2O2��Ӧ����Cu2����H2O���Ȼ�ѧ����ʽΪ______��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
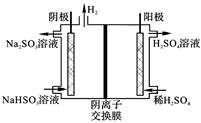
�ҹ���ҵ����Ҫ�����������ַ�������β���еĺ�������
| ����1 | ȼú�м���ʯ��ʯ����SO2ת��ΪCaSO3��������ΪCaSO4 |
| ����2 | �ð�ˮ��SO2ת��ΪNH4HSO3��������Ϊ(NH4)2SO4 |
| ����3 | ��������ˮú����SO2��ԭΪS |
| ����4 | ��Na2SO3��Һ����SO2���ٵ��ת��ΪH2SO4 |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com