
����Ŀ��5�ֹ�������A��B��C��D��E���±��в�ͬ������������ɣ����Ǿ�������ˮ��

�ֱ�ȡ���ǵ�ˮ��Һ����ʵ�飬������£�
��C��E��Һ�Լ��ԣ�A��B��D��Һ�����ԣ�0.1mol��L��E��ҺpH��13��
��B��Һ��E��Һ��Ϻ�������ɫ������ͬʱ�����������壻
������C��Һ��D��Һ��Ϻ������ɫ����������C��Һ��D��Һ��Ϻ�������
�ܽ�38.4 g CuƬͶ��װ������D��Һ���Թ��У�Cu���ܽ⣬�ٵμ�1.6 mol��L��1ϡH2SO4��Cu���ܽ⣬�ܿڸ����к���ɫ������֡�
��1���ݴ��ƶ�C��D�Ļ�ѧʽΪ��C______________��D_______________��
��2��д��������з�����Ӧ�����ӷ�Ӧ����ʽ____________________________��
��3�����������Ҫ��CuƬ��ȫ�ܽ⣬���ټ���ϡH2SO4�������____________mL��
��4������ȷ������ҺΪB��______________(����ĸ��ţ���
���𰸡�Ba(OH��2Al(NO3��32Fe3++3CO32-+3H2O=2Fe(OH��3��+3CO2��500A
��������
����������Ϣ��֪��C��E��Һ�Լ��ԣ���Һ����Ϊ����Һ��ǿ�������Σ�A��B��D��Һ�����ԣ�0.1mol��L��E��ҺpH��13����E�����������ˮ�⣬�������ӹ����֪��E����̼������ӣ�������ӹ��棬Eֻ��Ϊ̼���ƣ�������ӹ����֪��CΪ������������B��Һ��E��Һ��Ϻ�������ɫ������ͬʱ�����������壬��B�к��������ӣ���������̼������ӷ���˫ˮ�ⷴӦ���ɶ�����̼�������������ɫ������������C��Һ��D��Һ��Ϻ������ɫ��������������������Һ��D��Һ��Ϻ�������˵��D�к��������ӣ��Ҳ��������������ܽ�38.4 g CuƬͶ��װ������D��Һ���Թ��У�Cu���ܽ⣬�ٵμ�1.6 mol��L��1ϡH2SO4��Cu���ܽ⣬�ܿڸ����к���ɫ������֣�˵��D�к�����������ӣ���DΪ����������ôAΪ����ͭ�����Ȼ�ͭ����BΪ�Ȼ�������������
(1)�����Ϸ�����֪��CΪBa(OH��2��DΪAl(NO3��3 �� (2) �����Ϊ̼���ƺ������ӵ�˫ˮ�ⷴӦ�����ӷ���ʽΪ��2Fe3++3CO32-+3H2O=2Fe(OH��3��+3CO2����(3)38.4��ͭ�����ʵ���Ϊ38.4/64=0.6mol��������з��������ӷ�Ӧ����ʽΪ��3Cu+8H++2NO3-=3Cu2++2NO��+4H2O����Ҫ��ͭƬ��ȫ�ܽ⣬��Ҫ�����ӵ����ʵ���Ϊ1.6mol�������ټ���ϡ����������ΪV����1.6��V��2=1.6��V=500mL���ɷ�����֪AΪ����ͭ�����Ȼ�ͭ����BΪ�Ȼ�������������A��B������ȷ����


 ��ְٷְټ�����Ԫ��ĩ���Ծ�ϵ�д�
��ְٷְټ�����Ԫ��ĩ���Ծ�ϵ�д� Сѧ��ĩ���Ծ�ϵ�д�
Сѧ��ĩ���Ծ�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ijͬѧ����ͼ��ʾװ����ȡ�����ı���֤���÷�Ӧ��ȡ����Ӧ����ش�

��1������A������Ϊ____________������ˮ�Ľ�ˮ��Ϊ_________��(����m������n��)��
��2����ȡ�屽�Ļ�ѧ����ʽΪ___________________��
��3����ƿ������NaOH��Һ��������_________��
��4����ʵ�鰲ȫ�ĽǶȷ�������ʵ��װ�ô���һ�����Ե�ȱ����ָ��_________��
��5����Ӧ������������ƿ�еμ�����������Һ�������Ȼ����__________(����������)������屽(�Ժ���������)��
��6�����ʵ��֤����ȡ�屽�ķ�Ӧ��ȡ����Ӧ___________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����������ʵ�飺�ٳ�ȥ����ֲ�����е�ˮ���ڻ��յ��CCl4��Һ�е�CCl4������ʳ�þƾ������в�ҩ��ȡ���е���Ч�ɷ֡�����ʵ����ȷ����������( )
A. ��Һ����ȡ������ B. ��ȡ������Һ
C. ������ȡ����Һ D. ��Һ��������ȡ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���л���H��һ����Ҫ�ĸ߷��ӻ���������ϳ�·��������

��֪��
��ش�����������
��1��A��������_______________��C���������������______________��
��2��д����Ӧ������A��B______________��C��D__________________��
��3��B��C�ķ�Ӧ�Լ��ͷ�Ӧ������______________________��
��4��D+E��F�ķ�Ӧ����ʽ��_________________��
��5��G�ķ���ʽ��____________________��
��6����������������F��ͬ���칹�干��__________��(�����������칹)��
a.������������ȡ��������������״�ṹ��b.��̼̼��������-C��COH�ṹ��
��7������������л��о�����Ҫ�������������![]() ��.CH3CHO��
��.CH3CHO��![]() -CHO�ϳɶ������
-CHO�ϳɶ������ ��·��(���Լ���ѡ)______________
��·��(���Լ���ѡ)______________
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��������ʵ��װ����ɶ�Ӧ��ʵ�飨����������ȥ�����ܴﵽʵ��Ŀ���ǣ�������
A.  ��ȡ��������
��ȡ��������
B.  ����NH3
����NH3
C.  ʯ�͵ķ���
ʯ�͵ķ���
D.  �Ƚ����ᡢ̼�ᡢ���ӵ�����ǿ��
�Ƚ����ᡢ̼�ᡢ���ӵ�����ǿ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ѧ���о���ѧϰС�����ʵ�飬������ȡSO2��̽��SO2��ijЩ���ʡ���ȡSO2��Ӧ�Ļ�ѧ����ʽΪ��Na2SO3+2H2SO4��Na2SO4+SO2��+H2O������������ͨ����ͼ��ʾװ�ã�
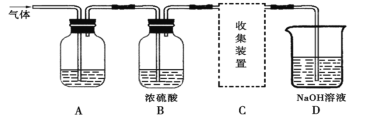
��ʵ��̽����
(1)��12.6g��Na2SO3��������Ũ���ᷴӦ���Ƶ�SO2�����Ϊ___________L����״����������ȡSO2�ķ�Ӧ________��ѡ��ǡ����ǡ���������ԭ��Ӧ��
(2)����Aװ�ü���SO2����Ư���ԣ���A�е���Һ��_________��
����Aװ�ü���SO2��һ�������������A�е���Һ��_________��
(3)Dװ���з����ķ�Ӧ�ǣ�д��ѧ����ʽ����____________________________________��
��ʵ�����ۣ�
(4)����ͼ�е�C�����ס�����ͬѧ��ѡ����ͼװ�ã��������ӷ�ʽ���в�ͬ�������ͬѧ��Ϊ��SO2����Ӧ��a��ͨ�뼯��ƿ�С���ͬѧ��Ϊ��SO2����Ӧ��b��ͨ�뼯��ƿ�С�����Ϊ________����ס����ҡ���ͬѧ�Ŀ�������ȷ�ġ�
����ϵʵ�ʣ�
(5)SO2�Ի�����Ӱ��ϴ�Ϊ�˼���SO2�Կ�������Ⱦ�������ӹ�ҵ�����ĽǶ����һ����Ч���еĴ�ʩ�������ֱ�������________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����Ǻϳ�ũҩ����Ҫ�м��壬��ϳ�·�����£�
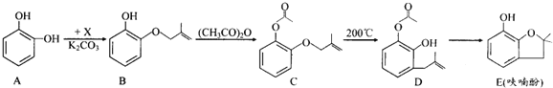
��1��A�ڿ����о��û�����ɫת��Ϊ�غ�ɫ����ԭ����____________��A�ں˴Ź�����������___________��塣
��2��B��C�ķ�Ӧ������_____________________��
��3����֪X�ķ���ʽΪC4H7Cl��д��A��B�Ļ�ѧ����ʽ��___________________��
��4��Ҫ������C��D�����˵��Լ���__________________________��
��5��B��ͬ���칹��ܶ࣬����������������______�֣�д�������ܷ���������Ӧ��ͬ���칹��Ľṹ��ʽ��__________(��дһ��)��
�ٱ������������������Ϊ��λ��ȡ���� �ۺ�������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��������ⷨ���Ѱ۲����ķ�Һ[���д���FeSO4��H2SO4������Fe2(SO4)3��TiOSO4]����������Ͳ�Ѫ�������������������������£�

��֪��TiOSO4������ˮ����ˮ�п��Ե���ΪTiO2+��SO42-��TiOSO4ˮ���TiO2xH2O����Ϊ���淴Ӧ������ṹ��ʽΪCH3CH(OH)COOH��
��ش�
��1��������з�������������Һ�������IJ�����________________________��
��2��������м��Ŀ��һ�ǻ�ԭ����Fe2(SO4)3������ʹ����TiOSO4ת��ΪTiO2xH2O��������ƽ���ƶ���ԭ�����͵õ�������ԭ��___________________________��
��3�����������ڿ�������������������������÷�Ӧ���������ͻ�ԭ�������ʵ���֮��Ϊ____________________________��
��4�������ӷ���ʽ���Ͳ�����м������ܵõ�����������ԭ��_________________��
��5������ܵ����ӷ���ʽ��_________________________________________��
��6������ޱ������һ������նȣ�ԭ��������������ˮ�Լ�___________________��
��7��Ϊ�ⶨ����������þ�����FeSO4��7H2O������������ȡ������Ʒa g������ϡ�������100.00 mL��Һ��ȡ��20.00 mL��Һ����KMnO4��Һ�ζ���������KMnO4����Ӧ����������0.1000 molL-1 KMnO4��Һ20.00 mL�����þ�����FeSO4��7H2O����������Ϊ______����a��ʾ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ҵ����COΪԭ�����������ѵķ�ӦΪ��3H2(g)��3CO(g) ![]() CH3OCH3(g)��CO2(g) ��H��a kJmol-1 T��ʱ����ʼʱ�ں����ܱ������м���һ������H2��CO��ʵ�����ݺͽ�����±�����ͼ��ʾ��
CH3OCH3(g)��CO2(g) ��H��a kJmol-1 T��ʱ����ʼʱ�ں����ܱ������м���һ������H2��CO��ʵ�����ݺͽ�����±�����ͼ��ʾ��
ʵ�� ��� | ���� ��� | ��ʼ���ʵ��� | ��ƽ��ʱ �ų����� | |
H2 | CO | |||
�� | 2L | 8mol | 8mol | 494 kJ |
�� | 2L | 4mol | 4mol | ���� |

��1��������Ӧƽ�ⳣ��K�ı���ʽΪ_____��
��2���������֪��a��______��b________1(�������������������)��
��3��ʵ����У���Ӧǰ10 min�ڵ�ƽ������v(H2)��_____��
��4������������ʹ������Ӧ�ķ�Ӧ����������ƽ��������Ӧ�����ƶ�����______ (��д�����ĸ)��
a����ʱ�����CH3OCH3����b�������������ݻ����䣬�ٳ���1 mol CO��1 mol H2
c���ʵ������¶� d�������������ݻ����䣬����1 mol ����
��5��T��ʱ���������к�1 molL-1 H2��2 molL-1 CO��2 molL-1 CH3OCH3��3 molL-1 CO2�����ʱv��________v��(�������������������)��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com