
����Ŀ��п�ǵ������������Ľ�������������������ͭ�����ִ���ҵ�ж��ڵ�ص������в���ĥ��Ĺ��ף�����������п����Ϊԭ���Ʊ�����п�Ĺ�ҵ���̡�
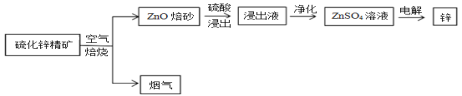
��1������Һ������пΪ���������� Fe3+��Fe2+��A13+��Cl-�����ʣ���Ӱ��п�ĵ�⣬�����ȥ�������������£�
�������������£���H2O2��Fe2+������Fe3+�����ӷ���ʽΪ___��
�ڽ�����Һ��pH����Ϊ5.5���ң�ʹ Fe3+��A13+�γɳ�������ȥ����ѡ�õ�����Լ�Ϊ___������ĸ��
A��NaOH B��NH3��H2O C��Zn(OH)2 D��H2SO4
����Ag2SO4�ɳ�ȥCl-��������Ӧ�����ӷ���ʽΪ______��
�ܵ�����������п�ĵ缫��ӦʽΪ___��
�������е�SO2�������̿�(��Ҫ�ɷ�MnO2�����ʽ���Ԫ��Fe��Al��Mg��)������Һ��Ӧ�Ʊ�MnSO4��H2O��
��2����֪��Ksp[Al(OH)3] =1��10��33��Ksp[Fe(OH)3] =3��10-39��pH =7.1ʱMn(OH)2��ʼ�����������£���ȥMnSO4��Һ�е�Fe3����Al3��(��ʹ��Ũ��С��1��10��6mol��L��1)���������ҺpH��ΧΪ___��
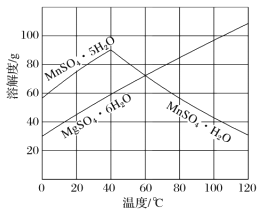
��3����ͼ���Կ�������MnSO4��MgSO4�����Һ�нᾧMnSO4��H2O���壬����ƽᾧ�¶ȷ�ΧΪ___��
��4��п�̼��Ե�ص��ܷ�ӦʽΪ��Zn��2MnO2��2H2O=2MnO(OH)��Zn(OH)2��������ӦʽΪ��_____��
���𰸡�2Fe2+��H2O2��2H+=2Fe3+��2H2O C Ag2SO4(s)��2Cl-(aq)![]() 2AgCl(s)��SO42-(aq) Zn2+��2e-=Zn 5.0<pH<7.1 ����60�� 2MnO2��2e-��2H2O=2MnO(OH)��2OH-
2AgCl(s)��SO42-(aq) Zn2+��2e-=Zn 5.0<pH<7.1 ����60�� 2MnO2��2e-��2H2O=2MnO(OH)��2OH-
��������
��п�����ڿ����б��յõ�ZnO�������������к��ж�������ZnO��ɰ���������������Һ�к���ZnSO4�ȣ�������õ�����п��Һ�����õ�Zn��
��1���������������£���H2O2��Fe2��������Fe3�����ɵ���غ㣬�������Ӳμӷ�Ӧ���������ԭΪˮ��
��ѡ����Լ������������ӣ��Ҳ������������ʣ�
���Ȼ����ܽ�ȸ�С����ȥCl-�����ӷ���ʽΪ��Ag2SO4(s)��2Cl-(aq)![]() 2AgCl(s)��SO42-(aq) ��
2AgCl(s)��SO42-(aq) ��
��Zn2��������ԭ��Ӧ�õ�Zn��������������
��2��pH=7.1ʱMn��OH��2��ʼ�����������£���ȥMnSO4��Һ�е�Fe3����Al3��������Al��OH��3��Fe��OH��3������ͬ����Al��OH��3���ܶȻ�����Fe��OH��3���ܶȻ���Al3+��ȫ����ʱFe3+һ����ȫ���������Al��OH��3��ȫ��ɳ���ʱ��pH������
��3����MnSO4��MgSO4�����Һ�нᾧMnSO4��H2O���壬����ͼ����Ϣ������60���Ժ�MnSO4��H2O���ܽ�ȼ�С����MgSO4��6H2O���ܽ��������˿��ƽᾧ�¶ȷ�Χ�Ǹ���60�棻
��4�����������������������϶������̵õ��ӷ�����ԭ��Ӧ��
��1���������������£���H2O2��Fe2��������Fe3�����ɵ���غ㣬�������Ӳμӷ�Ӧ���������ԭΪˮ����Ӧ���ӷ���ʽΪ��2Fe2��+H2O2+2H��=2Fe3��+2H2O��
��ѡ����Լ������������ӣ��Ҳ������������ʣ����������������Һ�������ӣ�NaOH��NH3��H2O��Zn��OH��2����������Һ�������ӣ���NaOH��NH3��H2O ���������������±�����ѡ��C��
���Ȼ����ܽ�ȸ�С����ȥCl-�����ӷ���ʽΪ��Ag2SO4(s)��2Cl-(aq)![]() 2AgCl(s)��SO42-(aq) ��
2AgCl(s)��SO42-(aq) ��
��Zn2��������ԭ��Ӧ�õ�Zn��������������п�ĵ缫��ӦʽΪ��Zn2��+2e��=Zn��
��2��pH=7.1ʱMn��OH��2��ʼ�����������£���ȥMnSO4��Һ�е�Fe3����Al3��������Al��OH��3��Fe��OH��3������ͬ����Al��OH��3���ܶȻ�����Fe��OH��3���ܶȻ���Al3+��ȫ����ʱFe3+һ����ȫ����������������ȫ��ɳ���ʱ��pH��Ksp[Al��OH��3]=1��10-33=c��Al3������c3��OH������c��Al3����=1��10-6mol��L-1����ã�c��OH����=1��10-9mol��L-1��c��H����=1��10-5mol��L-1����Һ��pH=5����pH��Χ�ǣ�5.0��pH��7.1��
��3����MnSO4��MgSO4�����Һ�нᾧMnSO4��H2O���壬����ͼ����Ϣ������60���Ժ�MnSO4��H2O���ܽ�ȼ�С����MgSO4��6H2O���ܽ��������MnSO4��H2O���ܽ��С��MgSO4��6H2O���ܽ�ȣ���˿��ƽᾧ�¶ȷ�Χ�Ǹ���60�棻
��4�����������������������϶������̵õ��ӷ�����ԭ��Ӧ���缫��ӦʽΪ2MnO2+2H2O+2e���T2MnO(OH)��2OH-��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����������ڸ��������µ�ͬ���칹����Ŀ��ȷ����
A.C4H10����������ͬ���칹����3��
B.���������C5H10O2���������ͬ���칹����5��
C.���������C5H10O����ȩ��ͬ���칹����3��
D.![]() ��һ�������5��
��һ�������5��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����ᡢ�������������ѧ�γ������������������־����������������ͭ��Ӧ��������ش��������⣺
(1)ϡ�����Cu��Ӧ������ϡ�����м���H2O2(��������������������ʱ��ԭ����Ϊˮ)�����ʹͭ˳���ܽ⣬�÷�Ӧ�����ӷ���ʽΪ_��
(2)��һ�������18mol��L-1��Ũ�����м������ͭƬ������ʹ֮��Ӧ������ԭ������Ϊ0.9mol����Ũ�����ʵ�����__(����������������������С����)100mL����ʹʣ���ͭƬ�����ܽ⣬�������м�����������Һ(��KNO3��Һ)����÷�Ӧ�����ӷ���ʽΪ__��
(3)������ͼ�����������ƶ���XΪ__(�����)��

A.Ũ���� B.Ũ���� C.Ũ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���ȿ�����������������ϩ���ֿ���������ȥ�����л��е�������ϩ�IJ���������(����)
A. �������ͨ��ʢ�����Ը��������Һ��ϴ��ƿ
B. �������ͨ��ʢ��������ˮ��ϴ��ƿ
C. �������ͨ��ʢ������ˮ��ϴ��ƿ
D. ��������������Ȼ�����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ͼ��ʾһЩ�����е�ijЩ�ṹ�����Ƿֱ���NaCl��CsCl���ɱ������ʯ��ʯī����ṹ�е�ijһ�ֵ�ijһ���֡�

��1���������ʯ����________(����ĸ��ţ���ͬ)������ÿ��̼ԭ����______��̼ԭ������Ҿ�����ȣ����ʯ����________���塣
��2������ʯī����_______��ÿ����������ռ�е�̼ԭ����ƽ��Ϊ_______����
��3������NaCl����_______��ÿ��Na+��Χ��������Ҿ�����ȵ�Na+��_______����
��4������CsCl����_______��������_______���壬ÿ��Cs+���_______Cl-���ڣ�
��5�������ɱ�����_______��������_______���壬ÿ��CO2������_______��CO2���ӽ��ڣ�
��6�����������������۵��ɸߵ��͵�����˳��Ϊ______________(����ĸ��Żش�)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ѧ�ҷ��ֶ�ұ�����е�⾫���ᴿ�ɽ��ߴ����Ʊ��ɱ�����ص���װ����ͼ��ʾ����Cu-Si�Ͻ�����Դ����950����������Һ���ν��е�⾫���� ����˵����ȷ����
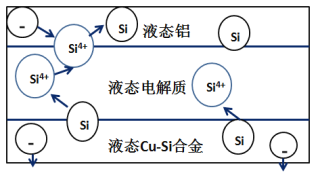
A.������Һ̬Cu-Si�Ͻ�����������Һ̬���缫
B.����Һ���ε�������ʹ�����ܹ����������������
C.�ڸ�Һ��������Cu������Si��������Si4+������Cu2+����ԭ
D.Һ̬���缫�븺����������Ϊ���ص�����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����Ȼ�����SnCl4�������л��ϳɵĴ������ڿ����м���ˮ������SnO2��xH2O��ʵ���������ڵ������۵�Ϊ231.9�棩��������Ӧ���Ʊ���ͬʱ��SnCl2���ɣ����÷�Ӧ�Ƿ��ȷ�Ӧ����ѡ�õ�������װ����ͼ��ʾ��
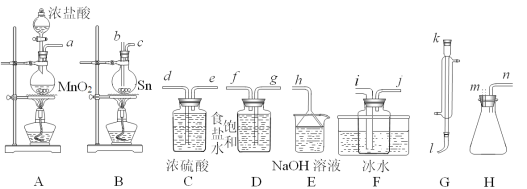
SnCl2��SnCl4��������������
���� | ��ɫ��״̬ | �۵�/�� | �е�/�� |
SnCl2 | ��ɫ���� | 246 | 623 |
SnCl4 | ��ɫҺ�� | -33 | 114 |
��1����֪SnCl2��SnCl4����������ͬ��SnCl2�۷е����SnCl4��ԭ���ǣ�___��
��2��ѡ��װ�ý�������(����ӿڵ���ĸ��װ�ÿ��ظ�ʹ�ã��ո�ɲ�����)��___
a��__��__��__��__��__��__��__��__��__��__��__��__��__��__��__��
��3����Ӧ��ʼʱ��Ӧ�ȵ�ȼװ��___���A����B������ͬ���ľƾ��ƣ���___ʱ���ٵ�Ȼװ��___�ľƾ��ƣ����ڷ�Ӧ�������Գ������ȣ���Ŀ����___��
��4���÷����Ƶõ�SnCl4�к�������SnCl2���ᴿSnCl4��ʵ��������___��
��5���ⶨ��Ʒ���ȡ�ȡag��Ʒ��������Ũ���ᣬ��������ˮϡ����250mL��ȡ20mLϡ����Һ����ƿ���μӼ��ε�����Һ����cmol��L-1I2��Һ�ζ����յ㣬���ĵζ���ҺV mL��
�ٸò�Ʒ��SnCl2����Ϊ___%�����ζ���Ӧ��Sn2����I2=Sn4����2I-��SnCl2����Է�������Ϊ��190��
��������ʱ����������ң���ò�Ʒ��SnCl4����__������ƫ��������ƫ����������Ӱ��������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����й��ڱ���������ȷ���ǣ� ��
A.�ӱ��Ľṹ��ʽ![]() �����������к���̼̼˫��
�����������к���̼̼˫��
B.���ķ���ʽΪC6H6������ʹ����KMnO4��Һ��ɫ
C.![]() �����ȴ�����3��
�����ȴ�����3��
D.�ڴ��������£�����Һ�巴Ӧ�����屽�������˼ӳɷ�Ӧ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
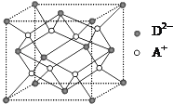
����Ŀ����A��B��C��D����Ԫ�أ�����AԪ�غ�BԪ�ص�ԭ�Ӷ���1��δ�ɶԵ��ӣ�A����B����һ�����Ӳ㣬Bԭ�ӵ�һ����������3p�����3p����ѳ�����Cԭ�ӵ�p�������3��δ�ɶԵ��ӣ�����̬�⻯����ˮ�е��ܽ����ͬ��Ԫ�����γɵ��⻯�������D������ϼۺ���ͻ��ϼ۵Ĵ�����Ϊ4��������������к�D����������Ϊ 40�����������������������������R����A��D��Ԫ���γɵ����ӻ��������A����D2��������֮��Ϊ2��1���ش��������⣺
(1)AԪ���γɵľ�������A2�ܶѻ���ʽ�����侧���ھ�������Ӧ����______(��д������������������������������������)��
(2)B���ĵ����Ų�ʽΪ____________����CB3������CԪ��ԭ�ӵ�ԭ�ӹ����������___________�ӻ���
(3)C���⻯��Ŀռ乹��Ϊ____________________�����⻯����ͬ��Ԫ�����γɵ��⻯���зе���ߵ�ԭ����________________________��
(4)BԪ�صĵ縺��_______DԪ�صĵ縺��(������������������������)����һ����ѧ����ʽ˵��___________��
(5)��ͼ��ʾ��R�γɵľ���ľ������辧�����ⳤΪa cm���Լ���R������ܶ�Ϊ_______��(�����ӵ�������NA��ʾ)
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com