
��֪�Ȼ�ѧ����ʽ��
��CO(g)�� O2(g)===CO2(g)����H����283.0 kJ��mol��1
O2(g)===CO2(g)����H����283.0 kJ��mol��1
��H2(g)�� O2(g)===H2O(g)����H����241.8 kJ��mol��1
O2(g)===H2O(g)����H����241.8 kJ��mol��1
������˵����ȷ����(����)
A��H2��ȼ����Ϊ241.8 kJ��mol��1
B���ɷ�Ӧ�١��ڿ�֪��ͼ��ʾ���Ȼ�ѧ����ʽΪCO(g)��H2O(g)===CO2(g)��H2(g)����H����41.2 kJ��mol��1

C��H2(g)ת���H2O(g)�Ļ�ѧ��Ӧһ���ͷ�����
D�����ݢ���֪��ӦH2(g)�� O2(g)===H2O(l)����H>��241.8 kJ��mol��1
O2(g)===H2O(l)����H>��241.8 kJ��mol��1
 �ľ�ͼ���ʱ�ȷ�ϵ�д�
�ľ�ͼ���ʱ�ȷ�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��1���������ڿ�����ȼ�գ���Ӧ�Ļ�ѧ����ʽ�� ������ˮ��Ӧ�����ӷ���ʽ�� ������������ˮ��Ӧ�����ӷ���ʽ�� ��
��2�����Ȼ�������Һ��п�ķ�Ӧ�У� ������������Ӧ�����ӷ���ʽ�� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ij��ѧ��Ӧ��������ͼ��ʾ����ͼ�ó����жϣ��������(����)
A��ͭ�Ǵ˷�Ӧ�Ĵ���
B���Ҵ������˻�ԭ��Ӧ
C��������M�Ļ�ѧʽΪC2H4O
D����Ӧ���к�ڽ���仯������
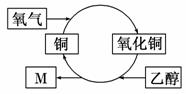
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��NA��ʾ������������ֵ������������ȷ���� �� ��
A����״���£�22.4L�ƾ����еķ�����Ϊ NA
B�����³�ѹ�£�10.6g Na2CO3���е�Na+����Ϊ0.2NA
C��ͨ��״���£�NA ��CO2����ռ�е����Ϊ22.4L
D�����ʵ���Ũ��Ϊ0.5 mol/L��MgCl2��Һ�У�����Cl������Ϊ NA
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��ͼΪʵ������ȡ����ˮ��װ��ʾ��ͼ������ͼʾ�ش���������
(1)ͼ�����Ե�����������
�� ��
(2)A������������ ��B������������ ��
(3) ʵ��ʱA�г���������ˮ�⣬����������� ���������� ��
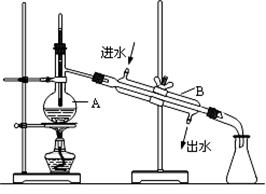
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ij��ѧ��Ӧ�ﵽ��ѧƽ�⣺A(g)��3B(g)2C(g)(��H<0)��������������¶Ƚ��ͣ�������������ȷ����(����)
A������Ӧ���ʺ��淴Ӧ���ʶ���С��ƽ��������Ӧ�����ƶ�
B������Ӧ���ʱ�С���淴Ӧ���ʼӴ�ƽ�����淴Ӧ�����ƶ�
C������Ӧ���ʼӴ��淴Ӧ���ʱ�С��ƽ��������Ӧ�����ƶ�
D������Ӧ���ʺ��淴Ӧ���ʶ���С��ƽ�����淴Ӧ�����ƶ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���ڵ��µĸֹܳ�����ͼ��ʾ�ķ������Ա�����ʹ�����ܸ�ʴ�����ڴ˷���������˵����ȷ����(����)
A��������X�IJ��Ͽ���Ϊͭ
B��������X�IJ��Ͽ���Ϊ��
C���ֹܸ���������pH���ܻ�����
D�����ַ�����Ϊ��ӵ���������������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��0.1 mol��L��1 CH3COOH��Һ�м���������CH3COONa����ʱ��������
(����)
A����Һ��pH���� B����Һ�е�c(H��)����
C����Һ�ĵ����������� D����Һ�е�c(OH��)��С
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��a L���ܱ��������һ���¶��½���2A��g��+B��g��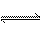 2C��g���Ŀ��淴Ӧ�������ڿ�ʼ�������и������ʣ��ڴﵽƽ��ʱ�淴Ӧ��������һ�������ǣ� ��
2C��g���Ŀ��淴Ӧ�������ڿ�ʼ�������и������ʣ��ڴﵽƽ��ʱ�淴Ӧ��������һ�������ǣ� ��
A.2 mol A��1 mol B B.1 mol A��1 mol B
C.1 mol A��2 mol B D.1 mol B��1 mol C
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com