
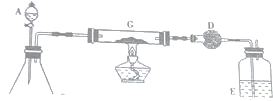
���� ����ʵ��װ��ͼ���ṩ��ҩƷ��֪��ʵ�����ö�������������ʹ˫��ˮ�ֽ������������C��������̼�����ɶ�����̼��ͬʱҲ������������D���ü�ʯ�����ղ����Ķ�����̼������Dװ�õ������ı仯ȷ��������̼��������������֪��Ʒ��̼Ԫ�ص�������Ϊ��ֹ�����е�ˮ����װ�ã�Ӱ��ʵ�����IJⶨ����Dװ�ú�����һ��װ��Ũ�����ϴ��ƿE��
��1������ѹǿ�������ͬ�����ж�װ�õ������ԣ�
��2��B����˫��ˮ�ڶ������̴�����������������
��3����������ķ�����֪��ʵ���в����Ķ�����̼������Ϊm2-m1��������Ʒ��̼������Ϊ$\frac{12}{44}��$��m2-m1��=$\frac{3}{11}$����m2-m1��������Ʒ����������ΪW-$\frac{3}{11}$����m2-m1�����ݴ˼�����������������
��4����Ϊ���õ������к���ˮ�֣���Ӱ��ʵ��IJⶨ��������������Cװ��ǰҪ���и���ݴ˴��⣻
��5���ⶨ��Ʒ����������������Ҳ����������ϡ���ᷴӦ����������������������������Ʒ����������������
��� �⣺����ʵ��װ��ͼ���ṩ��ҩƷ��֪��ʵ�����ö�������������ʹ˫��ˮ�ֽ������������C��������̼�����ɶ�����̼��ͬʱҲ������������D���ü�ʯ�����ղ����Ķ�����̼������Dװ�õ������ı仯ȷ��������̼��������������֪��Ʒ��̼Ԫ�ص�������Ϊ��ֹ�����е�ˮ����װ�ã�Ӱ��ʵ�����IJⶨ����Dװ�ú�����һ��װ��Ũ�����ϴ��ƿE��
��1������ѹǿ�������ͬ�����ж�װ�õ������ԣ������Ϊ���رշ�Һ©���Ļ���������ƿ�ȣ���E���������ݲ��������������������ã�
�ʴ�Ϊ���رշ�Һ©���Ļ���������ƿ�ȣ���E���������ݲ��������������������ã�
��2��B����˫��ˮ�ڶ������̴�������������������Ӧ����ʽΪ 2H2O2$\frac{\underline{\;MnO_2\;}}{\;}$2H2O+O2����
�ʴ�Ϊ��2H2O2$\frac{\underline{\;MnO_2\;}}{\;}$2H2O+O2����
��3����������ķ�����֪��ʵ���в����Ķ�����̼������Ϊm2-m1��������Ʒ��̼������Ϊ$\frac{12}{44}��$��m2-m1��=$\frac{3}{11}$����m2-m1��������Ʒ����������ΪW-$\frac{3}{11}$����m2-m1����R����������������Ϊ$\frac{W-\frac{3}{11}��{m}_{2}-{m}_{1}��}{W}$��100%��
�ʴ�Ϊ��$\frac{W-\frac{3}{11}��{m}_{2}-{m}_{1}��}{W}$��100%��
��4����Ϊ���õ������к���ˮ�֣���Ӱ��ʵ��IJⶨ��������������Cװ��ǰҪ���и������B��C֮������װ�ÿ����Ǹ���ܣ�����ʢ�ŵ�ҩƷ�Ǽ�ʯ�ң�
�ʴ�Ϊ������ܣ���ʯ�ң�
��5���ⶨ��Ʒ����������������Ҳ������һ����������Ʒ��ϡ���ᷴӦ����������������������������Ʒ����������������
�ʴ�Ϊ����һ����������Ʒ��ϡ���ᷴӦ����������������������������Ʒ����������������
���� ���������̼��������Ӧ̽���������������������������Ǹ߿��еij������ͣ������е��Ѷȵ����⣬�����ۺ���ǿ�����ض�ѧ��������������ѵ��������������ѧ���淶�Ͻ���ʵ����ơ�����������


 һŵ��ҵ�����ҵ���ּ�����������������ϵ�д�
һŵ��ҵ�����ҵ���ּ�����������������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
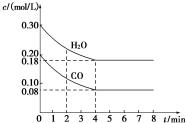 ��һ���Ϊ1L���ܱ������У�ͨ��һ������CO��H2O����T1��ʱ�������·�Ӧ��CO ��g��+H2O��g��?CO2��g��+H2��g����H��O��CO��H2OŨ�ȱ仯��ͼ����
��һ���Ϊ1L���ܱ������У�ͨ��һ������CO��H2O����T1��ʱ�������·�Ӧ��CO ��g��+H2O��g��?CO2��g��+H2��g����H��O��CO��H2OŨ�ȱ仯��ͼ����| ʱ�䣨min�� | CO | H2O | CO2 | H2 |
| 0 | 0.200 | 0.300 | 0 | 0 |
| 2 | 0.138 | 0.238 | 0.062 | 0.062 |
| 3 | C1 | C2 | C3 | C3 |
| 4 | C1 | C2 | C3 | C3 |
| 5 | 0.116 | 0.216 | 0.084 | C4 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
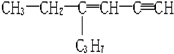 �������к���������ṹ��̼ԭ�ӣ�������̼ԭ�ӣ���Ϊx����ͬһֱ���ϵ�̼ԭ����Ϊyһ����ͬһƽ���ϵ�̼ԭ����Ϊz����x��y��z�ֱ�Ϊ��������
�������к���������ṹ��̼ԭ�ӣ�������̼ԭ�ӣ���Ϊx����ͬһֱ���ϵ�̼ԭ����Ϊyһ����ͬһƽ���ϵ�̼ԭ����Ϊz����x��y��z�ֱ�Ϊ��������| A�� | 5��6��4 | B�� | 5��3��6 | C�� | 5��4��8 | D�� | 5��3��5 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | 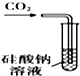 ��װ�ã�������֤��̼������Աȹ���ǿ | |
| B�� | 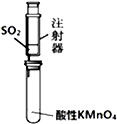 ��װ�ã���������������������� | |
| C�� | 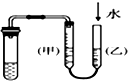 ��װ�ã���ͼʾ�ķ������ܼ���װ�õ������� | |
| D�� |  ��װ�ã��ȴӢٿڽ�������������̼���ٴӢڿڽ��������ռ����� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| �� | �� | �� | �� |
| 0.1mol•L-1 NaOH��Һ | pH=11NaOH��Һ | 0.1mo•L-1 CH3COOH ��Һ | pH=3 CH3COOH ��Һ |
| A�� | ����ܻ�ϣ�����Һ�����ԣ���������Һ������Ũ�ȿ���Ϊ��c��CH3COO-����c��H+����c��Na+����c��OH-�� | |
| B�� | ��ˮ�������c��OH-�����٣��� | |
| C�� | ��ϡ�͵�ԭ����100����pH�����ͬ | |
| D�� | ����ۻ�ϣ�����ҺpH=7����V��NaOH����V��CH3COOH�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | 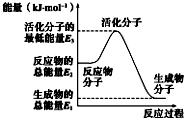 ��Ӧ���е������仯��ͼ��ʾ�����H2=E1-E3 | |
| B�� | N2��ȼ����Ϊ180kJ•mol-1 | |
| C�� | �ɷ�Ӧ��֪���¶�һ���������£���һ�����ܱ�������ͨ��1 mol N2�� 3 mol H2����Ӧ��ų�������ΪQ1 kJ����ͨ��2 mol N2�� 6 mol H2��Ӧ��ų�������ΪQ2 kJ����184.8��Q2��2Q1 | |
| D�� | ���Ĵ�������ӦΪ 4NH3��g��+5O2��g���T4NO��g��+6H2O��g����H=+906 kJ•mol-1 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| �� | �� | �� | ������ | |
| A | H2SO4 | Na2CO3 | NaCl | CO2 |
| B | HCl | NaOH | NaHCO3 | CaO |
| C | CO2 | NH3•H2O | Na2CO3 | CuO |
| D | CH3COOH | KOH | KCl | KClO3 |
| A�� | A | B�� | B | C�� | C | D�� | D |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | 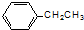 �һ��� �һ��� | B�� | 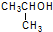 1-���Ҵ� 1-���Ҵ� | ||
| C�� | 2-��-1-��Ȳ | D�� | 2��3��3-�������� |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com