
����Ŀ��ij��ѧ��ȤС����̽���������Ļ�����IJ������ʡ�����������֪��Sn���۵�231�棻SnCl2�ױ�����������ˮ�⣻Sn(OH)2�������ֽ⣬SnCl4������Ϊ��ɫҺ�壬�۵㣭33�棬�е�114.1�棬��ش��������⣺
��1����С�������������Ʊ�SnSO4���壺

�ٲ����������ʵ������Ϊ______________________________________________��
�ڹ��˲����в�����ʹ�õ�ע������Ϊ____________________________��
�۲�����Ϊ������ϴ�ӡ����������жϳ����Ƿ�ϴ�Ӹɾ���____________________��
��2�������ڵ��������������Ʊ�SnCl4���ṩ��װ�����£�

��װ�â�Ϊ�����ܣ���ˮ����________���롣
�����ô�дӢ����ĸ��������˳��������װ����_________________________________________��
����ͬѧָ���������ӵ�ʵ��װ�ô��ڲ��㣬����֮��Ϊ______________________��
��3���ⶨ�������������IJ��裺ȡ����1.226g���������У����������FeCl3��Һ������0.1000mol��L1 K2Cr2O7��Һ�ζ�Fe2+������K2Cr2O7��Һ32.00mL�������ķ�Ӧ��6FeCl2+K2Cr2O7+
14HCl===6FeCl3+2KCl+2CrCl3+7H2O�������۵���������Ϊ(���ʲ����뷴Ӧ)____________��
���𰸡���1�����������������ƾ��ơ����ż�(�����Ȧ������̨)��������������һ����б�ǣ�����ʱ�ջ���������ڲ������ϣ������δ����ڶ����ֽ�ϣ���ȡϴ��Һ�������Թ��У�������HNO3�ữ��AgNO3��Һ���������ԣ�������֤��ϴ�Ӹɾ�������ϴ�Ӳ��ɾ�����2����Q����BJIKACDGHE(F)����ȱ��β������װ�ã�3��93��18%
��������
���⣨1��������Һ�л�����ʵľ��壬����ͨ�������ᾧ�õ�����˲����������ʵ������Ϊ�������������ƾ��ơ����ż�(�����Ȧ������̨)����ʴ�Ϊ���������������ƾ��ơ����ż�(�����Ȧ������̨)�����
�����˲�����ʹ�ò�����ʱҪע�⣺�������һ����б�ǣ�����ʱ�ջ���������ڲ������ϣ������δ����ڶ����ֽ�ϣ��ʴ�Ϊ���������һ����б�ǣ�����ʱ�ջ���������ڲ������ϣ������δ����ڶ����ֽ�ϣ�
������Iǰ�Ĺ��ˣ��õ�����Һ�к��������ӣ��жϳ����Ƿ�ϴ�Ӹɾ���ֻ���жϳ������Ƿ������������Ӽ��ɣ�������ȡϴ��Һ�������Թ��У�������HNO3�ữ��AgNO3��Һ���������ԣ�������֤��ϴ�Ӹɾ�������ϴ�Ӳ��ɾ����ʴ�Ϊ��ȡϴ��Һ�������Թ��У�������HNO3�ữ��AgNO3��Һ���������ԣ�������֤��ϴ�Ӹɾ�������ϴ�Ӳ��ɾ���
��2����ʹ��������ʱ����ȴˮӦ���½��ϳ���ˮ����Q���룬�ʴ�Ϊ��Q��
�������ڵ��������������Ʊ�SnCl4������Ҫ�Ʊ������������Ӧ��ѡ��װ�������������ȳ��Ȼ��⣬�ٸ��Ȼ���������ͨ�����з�Ӧ����SnCl4������SnCl4���������ʿ�֪��SnCl4�ķе�ϵͣ���������ʹ֮�����������ռ����ɵ�SnCl4��Ϊ�˷�ֹ���ˮ�����Ƚ���װ�ã��������һ������װ��������������װ������˳��ΪBJIKACDGHE(F)���ʴ�Ϊ��BJIKACDGHE(F)��
����Ӧ�е��������ܲ���ȫ��Ӧ������ɿ�����Ⱦ���ʴ�Ϊ��ȱ��β������װ�ã�
��3����Sn+2HCl��SnCl2+H2������
SnCl2+2FeCl3=SnCl4+2FeCl2����
6FeCl2+K2Cr2O7+14HCl��6FeCl3+2KCl+ 2CrCl3+7H2O��6Sn��K2Cr2O7��
�ɷ���ʽ�٢ڢ���֪K2Cr2O7��6FeCl2��3SnCl2��3Sn��
n(Sn)=3n(K2Cr2O7)=3��0��1000mol/L��0��032L=0��0096mol��
m(Sn)="n(Sn)��M(Sn)=" 0��0096mol��119g/mol= 1��1424g��
������Ʒ��������������=![]() ��100%=
��100%=![]() ��100% =93��18%���ʴ�Ϊ��93��18%��
��100% =93��18%���ʴ�Ϊ��93��18%��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����1��ijAl2��SO4��3��ҺVmL�к���a��Al3+,ȡ��![]() mL��Һϡ�ͳ�3VmL����������ӵ����ʵ���Ũ��Ϊ_________��
mL��Һϡ�ͳ�3VmL����������ӵ����ʵ���Ũ��Ϊ_________��
��2���ڱ�״���£�CO��CO2������������Ϊ36g�����Ϊ22.4L��������CO2��ռ�����Ϊ______��CO��ռ������Ϊ______��
��3��ij���������õ�Ӫ��Һ��Ҫ��KCl��K2SO4��NH4Cl���ֹ���ԭ�ϵ����ʵ���֮��Ϊ1:4:8��
�����Ƹ�Ӫ��Һ��c(NH4+)=0.016mol/L����Һ��c(K+)=_______________��
��������(NH4)2SO4��KCl�����Ƹ�Ӫ��Һ����(NH4)2SO4��KCl���ʵ���֮��Ϊ________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����й����Ȼ�ѧ��Ӧ����������ȷ����
A. HCl��NaOH��Ӧ���к��ȡ�H����57.3 kJ��mol1����H2SO4��Ca(OH)2��Ӧ���к��ȡ�H=2��(��57.3)kJ��mol1
B. ����ı�ȼ���Ȧ�H����890.3 kJ��mol1����CH4(g)��2O2(g)��CO2(g)��2H2O(g) ��H����890.3 kJ��mol1
C. ��֪��500�桢30MPa�£�N2(g)��3H2(g)![]() 2NH3(g) ��H����92.4kJ��mol��1����1.5 mol H2������N2�ڴ������³�ַ�Ӧ���ų�����46.2 kJ
2NH3(g) ��H����92.4kJ��mol��1����1.5 mol H2������N2�ڴ������³�ַ�Ӧ���ų�����46.2 kJ
D. CO(g)��ȼ������283.0kJ��mol1����2CO2(g) ===2CO(g)+O2(g)��Ӧ�ġ�H��+566.0 kJ��mol1
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ij�¶��£��ڼס��ҡ��������ĸ������ܱ�������Ͷ��H2��I2��������Ӧ:H2(g)+I2(g) ![]() 2HI(g)����Ӧ��ϵ�и�����Ũ�ȵ��й��������¡�
2HI(g)����Ӧ��ϵ�и�����Ũ�ȵ��й��������¡�
���� | ��ʼŨ�� | ƽ��Ũ�� | |
c(H2)/(mol��L-1) | c(I2)/(mol��L-1) | c(HI)/(mol��L-1) | |
�� | 0.01 | 0.01 | 0.004 |
�� | 0.01 | 0.02 | a |
�� | 0.02 | 0.01 | b |
�� | 0.02 | 0.02 | c |
�����жϲ���ȷ����
A. HI��ƽ��Ũ��:a=b>0.004��c=0.008 B. ƽ��ʱ��H2��ת����:��>��
C. ƽ��ʱ������H2��ת���ʴ���20% D. ���������£��÷�Ӧ��ƽ�ⳣ��K=0.25
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����й����Ҵ��������˵���У���ȷ����
A.�Ҵ�����������ж�����C=O��B.�Ҵ������ụΪͬ���칹��
C.�Ҵ����������ʹ��ɫʯ����Һ���D.�Ҵ��������֮�����ܽ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�������е����ⳣ�漰��ѧ֪ʶ�����й��̲��漰��ѧ�仯����(����)
A. ��ʳ�׳�ȥůƿ�ڵ�ˮ��
B. ��������Һ�м�������隣�����Һ��������ɫ����
C. �ⵥ�������۱���
D. ��75%���Ҵ���Һ����Ƥ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������ʽΪC9H18O2����������������ˮ�⣬���õ�����ʹ�����ͬ�����¶����������ܶ���ͬ�����ϴ�����������ͬ���칹��ĿΪ
A. 2 B. 8 C. 10 D. 16
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��CO��H2��CH3OH���������Դ��
��1����֪���ֻ�ѧ�������������£�
��ѧ�� | C | O=O | C=O | C-O |
E/(kJ mol-1) | 958.5 | 497 | 745 | 351 |
2CO(g) +O2(g)==2CO2(g) ![]() H1 H2O(g)+CO(g)==H2(g) + CO2(g)
H1 H2O(g)+CO(g)==H2(g) + CO2(g) ![]() H2 = -41 kJmol-1
H2 = -41 kJmol-1
CH3OH(g)+ 3/2O2(g)==CO2(g)+2H2O(g) ![]() H3 = -660kJmol-1
H3 = -660kJmol-1
����H1=_____ kJmol-1����ӦCO(g)+2H2(g)![]() CH3OH(g)����H=_____ kJmol-1��
CH3OH(g)����H=_____ kJmol-1��
��2��һ�������£����ݻ�Ϊ2 L���ܱ�����Q�г���a mol CO��b molH2�ϳɼ״���CO(g) +2H2(g) ![]() CH3OH(g)�����ƽ��ʱ���������CH3OH������ٷֺ������¶ȡ� ѹǿ֮��Ĺ�ϵ��ͼ1��ʾ��ͼ2��ʾ��һ���¶��£�H2��ƽ��ת�����뷴Ӧ��ʼʱ���ַ�Ӧ���Ͷ�����ʵ���֮�ȣ���X��ʾ����ѹǿ֮��Ĺ�ϵ��
CH3OH(g)�����ƽ��ʱ���������CH3OH������ٷֺ������¶ȡ� ѹǿ֮��Ĺ�ϵ��ͼ1��ʾ��ͼ2��ʾ��һ���¶��£�H2��ƽ��ת�����뷴Ӧ��ʼʱ���ַ�Ӧ���Ͷ�����ʵ���֮�ȣ���X��ʾ����ѹǿ֮��Ĺ�ϵ��
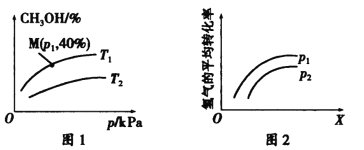
��ѹǿ��ͬʱ���¶�ΪT1��T2ʱ����Ӧ�ﵽƽ������Ҫ��ʱ��ֱ�Ϊt1��t2�������֮�����Դ�СΪt1___ t2(����>������<������=��������ȷ����)��
��P1_____P2(����>������<������=��������ȷ����)��
����a =2��b=4����ѹǿΪP1���¶�ΪT1ʱ�÷�Ӧ��ƽ�ⳣ��K=______________��
������ѹǿΪP1���¶�ΪT1ʱ����Q������ͬʱ��������ʵ�����CO��H2��CH3OH�������壬��Ӧ��ʼʱ��v(CH3OH)��_____v(CH3OH)��(����>������<������=��������ȷ����)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����֪CO2�����Һ������Ӧʱ�����η����������ӷ�Ӧ��CO2��2OH����CO32����H2O��CO2��CO32����H2O��2HCO
��1����0.01mol�������Ƶ�ʯ��ˮ��ͨ��CO2���õ�0.4g��ɫ��������ͨ��CO2�����ʵ���Ϊ______mol��______mol��
��2������NaOH��Ba(OH)2�����Һ100mL����֪����Һ��c(OH��)=1mol/L������Һ�л���ͨ��CO2���壨��Һ����仯���Բ��ƣ���
�ٵ�����ͨ��CO2�������Ϊ0.56L����״̬��ʱ���ɵij�����࣬��ԭ��Һ���������ƺ��������������ʵ���Ũ�ȸ�Ϊ���٣�__________________
�ڵ�ͨ���CO2���������Ϊ2.24L����״̬��ʱ����Һ�������ӣ�OH�����⣩�����ʵ���Ũ���Ƕ��٣�______________
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com