
����Ŀ����1����֪���� CH3OH(g)��H2O(g)===CO2(g)��3H2(g)����H����49.0 kJ/mol
�� CH3OH(g)��3/2O2(g)===CO2(g)��2H2O(g)����H����192.9 kJ/mol
����������ʽ��֪��CH3OH��ȼ����________(����������������������С����)192.9 kJ/mol��
��֪ˮ��������Ϊ44 kJ/mol�����ʾ����ȼ���ȵ��Ȼ�ѧ����ʽΪ__________��
��2����CO2��NH3Ϊԭ�Ͽɺϳɻ�������[��ѧʽΪCO(NH2)2]��
��֪���� 2NH3(g)��CO2(g)===NH2CO2NH4(s)����H����159.5 kJ/mol
��NH2CO2NH4(s)===CO(NH2)2(s)��H2O(g)����H����116.5 kJ/mol
��H2O(l)===H2O(g)����H����44.0 kJ/mol
д��CO2��NH3�ϳ����غ�Һ̬ˮ���Ȼ�ѧ����ʽ��______��
��3����֪���� Fe(s)��1/2O2(g)===FeO(s)����H1����272.0 kJ/mol
�� 2Al(s)��3/2O2(g)===Al2O3(s)����H2����1675.7 kJ/mol
Al��FeO�������ȷ�Ӧ���Ȼ�ѧ����ʽ��______��ijͬѧ��Ϊ�����ȷ�Ӧ�����ڹ�ҵ����������ж���______(������������������)�����������_____��
���𰸡����� H2(g)��1/2O2(g)===H2O(l)����H��-124.6 kJ/mol 2NH3(g)��CO2(g)===CO(NH2)2(s)��H2O(l)����H����87.0 kJ/mol 3FeO(s)��2Al(s)===Al2O3(s)��3Fe(s)����H����859.7 kJ/mol ���� �÷�Ӧ�������������Ĵ����������ɱ��ϸ�
��������
�Ȼ�ѧ��Ӧ�ͷŵ������뷴Ӧ�Ĺ����أ������ʵ�ʼ̬����̬�йأ����ø�˹���ɽ��м��㣻
��1��CH3OH��ȼ����Ϊ1molҺ̬�״���ȫȼ�������ȶ����������ͷŵ��������Աȷ�Ӧ������ȼ���ȴ���192.9kJ/mol����֪ˮ��������Ϊ44 kJ/mol������H2O(g)= H2O(l) ��H����44 kJ/mol�����ݸ�˹���ɣ�![]() +���ɵ�H2(g)��1/2O2(g)=H2O(l) ��H��-124.6 kJ/mol��
+���ɵ�H2(g)��1/2O2(g)=H2O(l) ��H��-124.6 kJ/mol��
��2�����ݸ�˹���ɣ���+��-�����ɵõ�2NH3(g)��CO2(g)=CO(NH2)2(s)��H2O(l) ��H����116.5��159.5-44.0=-87.0 kJ/mol��
��3�����ݸ�˹���ɣ���-�١�3�ɵ�2Al(s)��3FeO(s) =Al2O3(s)+3Fe(s) ��H����859.7 kJ/mol�����ȷ�Ӧ�������ڹ�ҵ��������÷�Ӧ��������Ҫ�϶����Դ���ɱ��ϸߡ�



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ƿ��ʢ������X���ι���ʢ��Һ��Y������ѹ��ͷ�ιܣ�ʹҺ��Y����ƿ�У�����һ��ɼ�С����a��������X��Һ��Y�������ǣ� ��
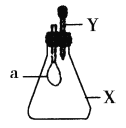
A.X��NH3��Y��ˮ
B.X��SO2��Y��NaOHŨ��Һ
C.X��CO2��Y��ϡ����
D.X��HCl��Y��NaNO3ϡ��Һ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��2017���ҹ�����ʹ�����ѺϽ���ϵĹ�����ĸ��ˮ����(Ti)���������ᡢ�������Ӧ�����������ܱ�������������������(��Ҫ�ɷ���FeO��TiO2)��ȡ�����ѵ���Ҫ����������ͼ������˵���������

A. ����I��̼����ԭ��
B. ����II��δ����������ԭ��Ӧ
C. ����III������������н��У���ֹ��������������
D. ����ϡ�����ȥ�������е�����þ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ͼ���¶Ⱥ�ѹǿ�Է�ӦX��Y![]() 2ZӰ���ʾ��ͼ��ͼ�к������ʾ�¶ȣ��������ʾƽ����������Z���������������������ȷ���ǣ� ��
2ZӰ���ʾ��ͼ��ͼ�к������ʾ�¶ȣ��������ʾƽ����������Z���������������������ȷ���ǣ� ��
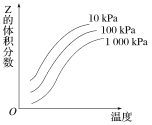
A.�������淴Ӧ������ӦΪ���ȷ�ӦB.X��Y��Z��Ϊ��̬
C.X��Y�����ֻ��һ��Ϊ��̬��ZΪ��̬D.������Ӧ���淴Ӧ����H��0
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��FeCl3����ѧʵ���ҳ��õ��Լ������������Ʊ������������塣
��1�������Ʊ�������������IJ���������ȷ����____________������ĸ����
A�����Ȼ�����Һ�еμ���������������ϡ��Һ
B����������Ȼ���������Һ
C���ڰ�ˮ�еμ��Ȼ���Ũ��Һ
D���ڷ�ˮ�еμӱ����Ȼ�����Һ�������Һ��ʺ��ɫ
��2��д���Ʊ�����������������ӷ���ʽ_______________��
��3�������뽺�������ص���_____________������ĸ����
A�������뺣�ڴ��γ�ɳ��
B��ʹ�������������������������ʹ��������ѪҺѸ�����̶�����ʧѪ
C��������˥�ߵȼ��������ѪҺ�ж���������ѪҺ����������
D���ڱ����Ȼ�����Һ�еμ�NaOH��Һ���������ɫ����
E��ұ���ø�ѹ���ȥ�̳�
��4������Һ�з����ᴿFe(OH)3����ķ�����_____________��
��5��������������������μ���ϡ������������������_____________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��NAΪ�����ӵ�������ֵ������˵����ȷ����( )
A. ��״���£�22.4 Lˮ���е�ˮ������ΪNA
B. ���³�ѹ�£�22 g CO2���е�CO2������Ϊ0.5NA
C. ��״���£�32 g O2��CO2�Ļ�����庬�е���ԭ����Ϊ2NA
D. 40 g NaOH�ܽ���1 Lˮ�У��õ���Һ�����ʵ���Ũ��Ϊ1 mol/L
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�������Ƕ�ij��Һ�������Ӽ��ķ����ͽ��ۣ�������ȷ����(����)
A. �ò�˿պȡ����ij��Һ������ɫ��Ӧ������ʻ�ɫ������Һһ����������Һ
B. ����������CaCl2��Һ��������ɫ����������Һ��һ�����д�����CO![]()
C. ����NaOH��Һ����Ȳ����д̼�����ζ�����壬����Һ��һ�����д�����NH![]()
D. �ȼ��������������ữ���ټ���AgNO3��Һ��������ɫ����������Һ��һ�����д�����Cl��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����֪�����������ת����
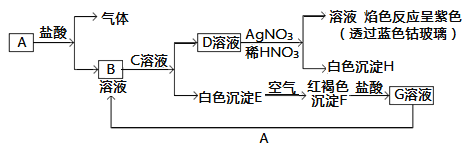
�Իش�
(1)д��B�Ļ�ѧʽ________��D�Ļ�ѧʽ________��
(2)д����Eת���F�Ļ�ѧ����ʽ________________________��
(3)д����KSCN����G��Һ�����ӷ���ʽ________________����G��Һ����A���й����ӷ�Ӧ����ʽ________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������a mol FeBr2����Һ�У�ͨ��x mol Cl2�����и���ΪͨCl2�����У���Һ�ڷ�����Ӧ�����ӷ���ʽ�����в���ȷ���ǣ� ��
A.x��0.4a��2Fe2++Cl2��2Fe3++2Cl-
B.x��0.6a��2Br��+Cl2��Br2+2Cl��
C.x=a��2Fe2��+2Br��+2Cl2��Br2+2Fe3��+4Cl��
D.x=1.5a��2Fe2��+4Br��+3Cl2��2Br2+2Fe3��+6Cl��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com