
����Ŀ��2019��3��21���ǵڶ�ʮ�߽�������ˮ����������ˮ��Դ���������÷�ˮ��ʡˮ��Դ����ǿ��ˮ�Ļ��������ѱ�Խ��Խ���������ע����֪��ij��ɫ��ˮ�п��ܺ���H+��NH4+��Fe3+��Al3+��Mg2+��Na+��NO3-��CO32-��SO42-�еļ��֣�Ϊ������ɷ֣��ֱ�ȡ��ˮ��Ʒ100![]() ������������ʵ�飬��������й�ͼ������ͼ��ʾ����ش��������⣺
������������ʵ�飬��������й�ͼ������ͼ��ʾ����ش��������⣺
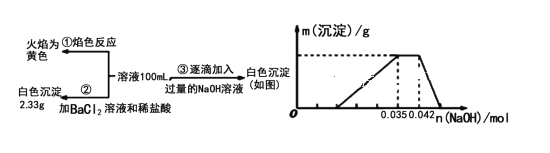
(1)��������3��ʵ����Է�����ˮ��һ�������ڵ���������_______��һ�����ڵ���������____________________��
(2)д��ʵ���ͼ���г����ﵽ��������������ٷ����仯�η�����Ӧ�����ӷ�Ӧ����ʽ��_________________________��
(3)����ͼ����ԭ��Һ��c(NH4+)��c(Al3+)�ı�ֵΪ__________�����ó��������������__________g��
(4)��ͨ��ʵ��ȷ��ԭ��ˮ��c(Na+)=0.14mol/L�����ж�ԭ��ˮ��NO3-�Ƿ����?__________(��������������������������ȷ����)�������ڣ�c(NO3-)=__________mol/L��(�������ڻ�ȷ����˿ղ���)��
���𰸡�CO32- Na+��H+��Al3+��NH4+ NH4++OH-=NH3��H2O 1��1 0.546 ���� 0.36
��������
(1)����ʵ�����ɫ��ӦΪ��ɫȷ������Na+������ʵ��ڼ���ϡHCl�ữ��BaCl2��Һ��������ɫ������ȷ������SO42-������ʵ��ۼ���NaOH��Һ����ʼ������˵������H+������H+��CO32-���ܴ������棬֤��������CO32-������������ɫ������֤�������в������ɫ������Fe3+��Ȼ��������ٱ仯����������ȫ��ʧ��֤������Al3+����Mg2+���ɼ�ͨ���ۿ�֤������H+��Al3+��NH4+����CO32-��Mg2+��Fe3+��Ȼ����ݷ�Ӧ���������ĵ�NaOH�����ʵ���ȷ���������ӵ����ʵ����Ķ��٣������Һ������ȷ���������ӵĴ��ڼ���Ũ�ȴ�С��
(1)����ʵ���ȷ������Na+������ʵ���ȷ������SO42-������ʵ���ȷ����H+��Al3+��NH4+��û��Fe3+��Mg2+����ΪCO32-��H+��Al3+���ܹ��棬����һ��������CO32-���ɼ��÷�ˮ��һ�������ڵ���������CO32-��һ�����ڵ���������Na+��H+��Al3+��NH4+��
(2)ʵ���ͼ���г����ﵽ��������������ٷ����仯�Σ���NH4+��OH-֮������ӷ�Ӧ�����ӷ���ʽΪ��NH4++OH-=NH3��H2O��
(3)����ͼ�ӿ�ʼ�γɳ����������ﵽ���ֵ��������ӦAl3++3OH-=Al(OH)3����n(Al3+)=![]() =0.007mol��n(NH4+)=0.042mol-0.035mol=0.007mol����Һ�������ͬ������ԭ��Һ��c(NH4+)��c(Al3+)�ı�ֵΪ1��1�����ó������������m[Al(OH)3]=0.007mol��78g/mol=0.546g��
=0.007mol��n(NH4+)=0.042mol-0.035mol=0.007mol����Һ�������ͬ������ԭ��Һ��c(NH4+)��c(Al3+)�ı�ֵΪ1��1�����ó������������m[Al(OH)3]=0.007mol��78g/mol=0.546g��
(4)�������ᱵ����������2.33g��n(SO42-)=n(BaSO4)=![]() =0.01mol�����ݵ���غ㣬�����ӵ�������ʵ���Ϊ0.01mol��2=0.02mol�������ӵ�������ʵ���Ϊn(H+)+n(Al3+)+ n(NH4+)+n(Na+)=0.014mol+0.007��3mol+0.007mol+0.14mol/L��0.1L=0.056mol��������������ڸ��������������ԭ��ˮ�д���NO3-��c(NO3-)=
=0.01mol�����ݵ���غ㣬�����ӵ�������ʵ���Ϊ0.01mol��2=0.02mol�������ӵ�������ʵ���Ϊn(H+)+n(Al3+)+ n(NH4+)+n(Na+)=0.014mol+0.007��3mol+0.007mol+0.14mol/L��0.1L=0.056mol��������������ڸ��������������ԭ��ˮ�д���NO3-��c(NO3-)=![]() =0.36mol/L��
=0.36mol/L��


 ������ҵ��ͬ����ϰ��ϵ�д�
������ҵ��ͬ����ϰ��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����1����һ�ֽ�������A����ɫ��Ӧ��ɫ�ʻ�ɫ���ܷ�����ͼ��ʾ�仯��

��ͼ�е���ɫ����B��_____________�ѧʽ��
��2���ƵĻ������У������ں��������ΪO2��Դ����______
��3��д����1����C��Һ�����ᷴӦ�Ļ�ѧ����ʽ��______
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ijУ����С��Ϊ�ⶨij̼���ƺ�̼�����ƻ������̼���Ƶ������������ס�������ͬѧ�ֱ�������������ʵ�飮
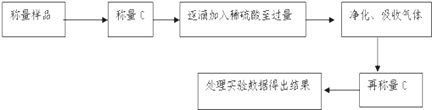
������������ͬѧ����������������ͼ��ʾ��ʵ�����̽���ʵ�飺

��1��ʵ��ʱ�������ᾧ�����У����˾ƾ����⣬��Ҫ�õ���������_______
��2����ͬѧ��Ϊ�������������������òٿأ�Ӧ��Ϊ�����������������ڲ����Ҳ�Ӱ��ⶨ��ȷ�ԣ�����Ϊ�Ի��_______��Ϊʲô___________________
��3����ʵ���в����Ʒ����Ϊ46.4g����������Ϊ40.95g����̼���Ƶ���������Ϊ_______��������3λ��Ч���֣�
��4�������ᾧ���������й���ɽ������̼���Ƶ���������____________����ƫ�� ƫС ��Ӱ�죩��
������������ͬѧ����Ҫʵ������ͼ���£�
������ͼ��ʾװ�ý���ʵ�飺
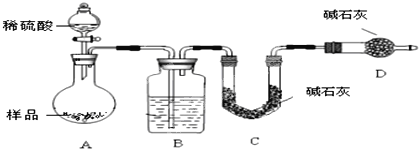
��5����C��װ��ʯ�������վ���������壮Dװ�õ�������_____________________��
��6���е�ͬѧ��ΪΪ�˼���ʵ�����ڷ�Ӧǰ��ͨ��N2����Ӧ��ͨ��N2��Ŀ����______________________________��
�����������������
��7����һ������Ʒ������ϡ���ᷴӦ������ͼװ�ò�������CO2����������B��Һ��ò���_________������ѡ����ѡ��ʹ��������С��
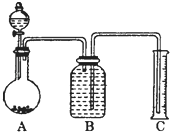
A������̼������Һ | B������̼��������Һ |
C����������������Һ | D����������ͭ��Һ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���������NaOH��Һ�зֱ�ͨ��CO2���塣��CO2��ͨ������ͬ���õ���ֲ�ͬ����ҺM������M����μ������ᣬ�������������V(CO2)�������������V(HCl)��ϵ��ͼ��(ע���ټ���CO2ȫ���ݳ�����ͼ��Oa<ab����ͼ��Oa=ab����ͼ��Oa>ab)������M��ֻ��1�����ʵ��ǣ� ��

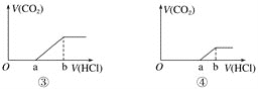
A.ֻ�Т�B.ֻ�Т�C.�ڢ�D.�٢�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����������ʵ��װ��ͼ�ش����⣺
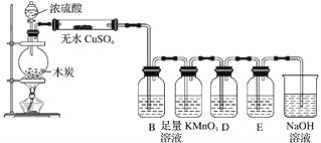
��1��ŨH2SO4��ľ̿�ڼ���ʱ������Ӧ�Ļ�ѧ����ʽ��__�������0.2mol����ת�ƣ����ڱ�״���²�������__��
��2������ͼʾ�е�װ�ü���������Ӧ��ȫ�����д���й�������Ӧ������Լ������ã���ˮCuSO4�Լ�������__��B�м�����Լ���__��������__������KMnO4��Һ������___��D�м�����Լ���__��������__��NaOH��Һ��������__��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����л�ѧ����ʽ����뷽��ʽ�У�������ȷ���ﷴӦ��ɫ�仯����
A.������ͨ����з�̪��Һ��ˮ�У���Һ��죺NH3��H2O![]() NH3��H2O
NH3��H2O![]() NH4����OH��
NH4����OH��
B.����FeCl3��Һ�����ˮ�б�Ϊ���ɫҺ�壺FeCl3��3H2O![]() Fe(OH)3(����)��3HCl
Fe(OH)3(����)��3HCl
C.��CuCl2��Һ�м������������ۣ���Һ����ɫ��Ϊdz��ɫ��Fe��CuCl2��Cu��FeCl2
D.�������ʹ�ú�Na2O2�ɵ���ɫ��Ϊ��ɫ��2Na2O2��2Na2O��O2��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ʵ�����Ʊ���������KaFeb(C2O4)c��xH2O�Ĺ����������£�
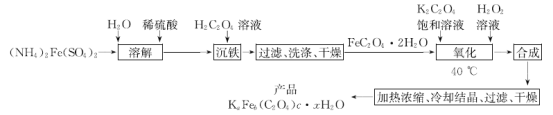
�ش��������⣺
(1)���ܽ���ʱ��Ϊʹ���õ�ˮ�в���O2�����õIJ���������_________________��
(2)��H2C2O4(����)��������ʱ����Ӧ�����ӷ���ʽΪ______________��
(3)FeC2O4��2H2O�ȷֽ������ռ��¶��йأ���N2�������ȷֽ�ʱ������IJ�����(������Ʒ��ʣ������/������Ʒ����ʼ������100��)���¶ȵĹ�ϵ��ͼ��ʾ����B��C�ı仯�У�������Ӧ�Ļ�ѧ����ʽΪ___________________��
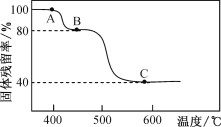
(4)��������ʱ���¶Ȳ��˳���40�棬��ԭ����_______��
(5)Ϊ�ⶨ��ƷKaFeb(C2O4)c��xH2O(��Ԫ��Ϊ��3��)����ɣ���ȡ��Ʒ0.2455g�������ܽ����0.02000 mol��L��1��KMnO4����Һ�ζ����յ㣬���ı���Һ30.00 mL���������ζ���C2O42���ı���Һ�м�������п�ۣ���������ɫ��ʧ������ϴ�ӣ���Һ��ϴ��Һ����0.02000 mol��L��1��KMnO4����Һ�ζ����յ㣬���ı���Һ5.00 mL����ò�Ʒ�Ļ�ѧʽΪ____________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��������������������������Ź㷺��Ӧ�ã�ij��ѧ��ȤС������ͼһװ��̽���������й����ʡ�
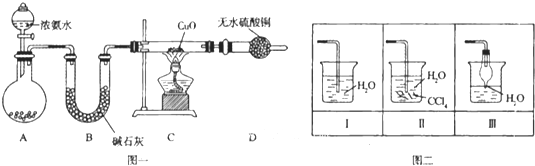
��1��װ��A����ƿ���Լ���ѡ�� �����������B��������
a����ʯ�� b��Ũ���� c����ʯ�� d���ռ���Һ
��2�����Ӻ�װ�ò�����װ�õ������Ժ�װ��ҩƷ��Ȼ��Ӧ�� ����I�������
�����������Բ����ƿ�м��백ˮ ����װ��C
��3��ʵ���й۲쵽C��CuO��ĩ��죬D����ˮ����ͭ���������ռ���һ�ֵ������壬��÷�Ӧ��ػ�ѧ����ʽΪ ,���÷�Ӧ֤���������� �ԣ�
��4����ʵ��ȱ��β������װ�ã�ͼ��������������β����װ���� ����װ���������
��5��������������ˮ������״���£���2.24L�İ�������ˮ���0.5L��Һ��������Һ�����ʵ���Ũ��Ϊ mol/L��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ij��ѧ�С���������װ�ý��в�ͬ��ʵ�顣����aΪ���ڹ�����������ң�b Ϊ����״ͭ˿��c��ʢ�б�ˮ��

��1������Aװ�����Ҵ��������������Ӧʵ�飬�������ӵ�װ����____________(�����)����װ����Ӧ�����Լ�____________����ʵ�鰲ȫ�Ƕȿ��ǣ�Aװ���Թ��г����뷴ӦҺ�⣬�������Ĺ���������____________��
��2����С��ͬѧ�����Ҵ���������ȩ��ʵ�飬��Ӧѡ�õ�װ����____________(�����)�������Ƶõ���ȩ��Һ����������Ӧ����ȷ�IJ���˳����____________(�����)��
�����Թ��е���3����ȩ��Һ
��һ����һ�ߵ���2%��ϡ��ˮ��ֱ����������ij���ǡ���ܽ�Ϊֹ
���������ˮ�У�ˮԡ����
���ڽྻ���Թ��м���1 mL 2%��AgNO3��Һ
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com