
ijͬѧ��ѧϰ�˰��������Ժ�������ȵ�˼��˼����
(1)����������һ��������ܷ�����һ��Ҳ�γ���Ȫ��
(2)�������л�ԭ�ԣ��ܷ���H2������ԭCuO�أ����������ʵ����ȡ������̽���������⣮����������Ļ����������о���
(��)��ȡ��������Ȫ�����̽��
1��д��ʵ����ȡ�����Ļ�ѧ����ʽ________��
2���ռ������ķ�����________��
3����Ȫ��һ�ֳ�������Ȼ����
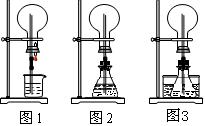
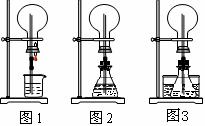
(1)ͼ1Ϊ��ѧ�̲��е���Ȫʵ��װ�ã���������˼����ֻҪ������ƿ��ѹǿ�벣����ˮ��ѹǿ�ĺ�С����ƿ���ѹǿ�Ϳ��Բ�����Ȫ�������������ͼ2��ͼ3��ʾ��װ�ã�

����ͼ2����ƿ�У��ֱ�����������������ʣ���Ӧ����ܲ�����Ȫ����________��
A��Cu��ϡ����
B��NaHCO3��NaOH��Һ
C��MnO2��ϡ����
D��Na2CO3��ϡ����
����ͼ3��ƿ�м����ӷ�����(��ƾ�)��ˮ���м�����ˮ���ټ������е��������������Ҳ��������Ȫ��ˮ���к��������ʿ�����________��
A��Ũ����
B��ʳ��
C����ʯ��
D������
(2)�����г�����������Ȫ����ɽ������ԭ��������________(�ӡ�ͼ1����ͼ2����ѡ��)װ�õ�ԭ�����ƣ�
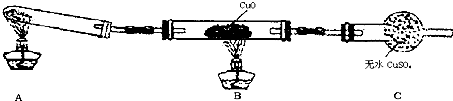
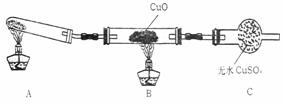
(��)��С����ijͬѧ�����������ʾ��ʵ��װ��(�гּ�β������װ��δ����)��̽�������Ļ�ԭ�ԣ�

(1)��װ�����������һ��ȱ�ݣ�Ϊ��֤ʵ������ȷ�ԣ��Ը�װ�õĸĽ���ʩ��________��
(2)���øĽ����װ�ý���ʵ�飬�۲쵽CuO��Ϊ��ɫ���ʣ���ˮCuSO4����ɫ��ͬʱ����һ������Ⱦ�����壮д��������CuO��Ӧ�Ļ�ѧ����ʽ________��
 ��ɢ˼ά�¿���ϵ�д�
��ɢ˼ά�¿���ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
| ||
| ||
| ||
| ||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
. ijͬѧ��ѧϰ�˰��������Ժ�������ȵ�˼��˼������1������������һ��������ܷ�����һ��Ҳ�γ���Ȫ����2���������л�ԭ�ԣ��ܷ���H2������ԭCuO�أ����������ʵ����ȡ������̽���������⡣����������Ļ����������о���
������ȡ��������Ȫ�����̽��
1.д��ʵ����ȡ�����Ļ�ѧ����ʽ ��
2.�ռ������ķ����� ��
3.��Ȫ��һ�ֳ�������Ȼ����
��1��ͼ1Ϊ��ѧ�̲��е���Ȫʵ��װ�á���������˼����ֻҪ������ƿ��ѹǿ�벣����ˮ��ѹǿ�ĺ�С����ƿ���ѹǿ�Ϳ��Բ�����Ȫ�������������ͼ2��ͼ3��ʾ��װ�á�
����ͼ2����ƿ�У��ֱ�����������������ʣ���Ӧ����ܲ�����Ȫ����__________��
A.Cu��ϡ���� B.NaHCO3��NaOH��Һ C.MnO2��ϡ���� D.Na2CO3��ϡ����
����ͼ3��ƿ�м����ӷ����ʣ���ƾ�����ˮ���м�����ˮ���ټ������е��������������Ҳ��������Ȫ��ˮ���к��������ʿ�����___________��
A.Ũ���� B.ʳ�� C.��ʯ�� D.����
��2�������г�����������Ȫ����ɽ������ԭ��������___________���ӡ�ͼ1����ͼ2����ѡ��װ�õ�ԭ�����ơ�
����С����ijͬѧ�����������ʾ��ʵ��
װ�ã��гּ�β������װ��δ��������̽������
�Ļ�ԭ�ԣ�
|
|
|
��2�����øĽ����װ�ý���ʵ�飬�۲쵽CuO��Ϊ��ɫ���ʣ���ˮCuSO4����ɫ��ͬʱ����һ������Ⱦ�����塣д��������CuO��Ӧ�Ļ�ѧ����ʽ ��

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ʦ����2009��2010ѧ���һ��ѧ����ĩ����ѧ������ ���ͣ�ʵ����
(15��)ijͬѧ��ѧϰ�˰��������Ժ�������ȵ�˼��˼������1������������һ��������ܷ�����һ��Ҳ�γ���Ȫ����2���������л�ԭ�ԣ��ܷ���H2������ԭCuO�أ����������ʵ����ȡ������̽���������⡣����������Ļ����������о���
������ȡ��������Ȫ�����̽��
1.д��ʵ����ȡ�����Ļ�ѧ����ʽ ��
2.�ռ������ķ����� ��
3.��Ȫ��һ�ֳ�������Ȼ����
��1��ͼ1Ϊ��ѧ�̲��е���Ȫʵ��װ�á���������˼����ֻҪ������ƿ��ѹǿ�벣����ˮ��ѹǿ�ĺ�С����ƿ���ѹǿ�Ϳ��Բ�����Ȫ�������������ͼ2��ͼ3��ʾ��װ�á�
����ͼ2����ƿ�У��ֱ�����������������ʣ���Ӧ����ܲ�����Ȫ����__________��
A.Cu��ϡ���� B.NaHCO3��NaOH��Һ C.MnO2��ϡ���� D.Na2CO3��ϡ����
����ͼ3��ƿ�м����ӷ����ʣ���ƾ�����ˮ���м�����ˮ���ټ������е��������������Ҳ��������Ȫ��ˮ���к��������ʿ�����___________��
A.Ũ���� B.ʳ�� C.��ʯ�� D.����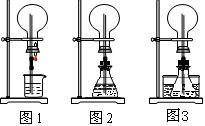
��2�������г�����������Ȫ����ɽ������ԭ��������___________���ӡ�ͼ1����ͼ2����ѡ��װ�õ�ԭ�����ơ�
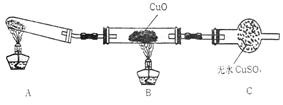
����С����ijͬѧ�����������ʾ��ʵ��
װ�ã��гּ�β������װ��δ��������̽������
�Ļ�ԭ�ԣ�
|
|
|
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
 ijͬѧ��ѧϰ�˰��������Ժ�������ȵ�˼��˼������1������������һ��������ܷ�����һ��Ҳ�γ���Ȫ����2���������л�ԭ�ԣ��ܷ���H2������ԭCuO�أ����������ʵ����ȡ������̽���������⡣����������Ļ����������о���
ijͬѧ��ѧϰ�˰��������Ժ�������ȵ�˼��˼������1������������һ��������ܷ�����һ��Ҳ�γ���Ȫ����2���������л�ԭ�ԣ��ܷ���H2������ԭCuO�أ����������ʵ����ȡ������̽���������⡣����������Ļ����������о���
������ȡ��������Ȫ�����̽��
1.д��ʵ����ȡ�����Ļ�ѧ����ʽ ��
2.�ռ������ķ����� ��
3.��Ȫ��һ�ֳ�������Ȼ����
��1��ͼ1Ϊ��ѧ�̲��е���Ȫʵ��װ�á���������˼����ֻҪ������ƿ��ѹǿ�벣����ˮ��ѹǿ�ĺ�С����ƿ���ѹǿ�Ϳ��Բ�����Ȫ�������������ͼ2��ͼ3��ʾ��װ�á�
����ͼ2����ƿ�У��ֱ�����������������ʣ���Ӧ����ܲ�����Ȫ����__________��
A.Cu��ϡ���� B.NaHCO3��NaOH��Һ C.MnO2��ϡ���� D.Na2CO3��ϡ����
����ͼ3��ƿ�м����ӷ����ʣ���ƾ�����ˮ���м�����ˮ���ټ������е��������������Ҳ��������Ȫ��ˮ���к��������ʿ�����___________��
A.Ũ���� B.ʳ�� C.��ʯ�� D.����
��2�������г�����������Ȫ����ɽ������ԭ��������___________���ӡ�ͼ1����ͼ2����ѡ��װ�õ�ԭ�����ơ�
 ����С����ijͬѧ�����������ʾ��ʵ��
����С����ijͬѧ�����������ʾ��ʵ��
װ�ã��гּ�β������װ��δ��������̽������
�Ļ�ԭ�ԣ�
��1����װ�����������һ��ȱ�ݣ�Ϊ��֤ʵ������ȷ�ԣ��Ը�װ�õĸĽ���ʩ�� ��
��2�����øĽ����װ�ý���ʵ�飬�۲쵽CuO��Ϊ��ɫ���ʣ���ˮCuSO4����ɫ��ͬʱ����һ������Ⱦ�����塣д��������CuO��Ӧ�Ļ�ѧ����ʽ ��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com