
����Ŀ���л���F�� ��Ϊһ�ָ߷�����֬����ϳ�·�����£�
��Ϊһ�ָ߷�����֬����ϳ�·�����£�

��֪����AΪ����ȩ��ͬϵ���������������Է�������Ϊ134��
��
��ش��������⣺
��1��X�Ļ�ѧ������_________________��
��2��E����F�ķ�Ӧ����Ϊ_________________��
��3��D�Ľṹ��ʽΪ_________________��
��4����B����C�Ļ�ѧ����ʽΪ_________________��
��5�������廯����Y��D��ͬϵ�Y��ͬ���칹�����뱥��Na2CO3��Һ��Ӧ�ų����壬������ֻ��1���������˴Ź���������ʾ��5�ֲ�ͬ��ѧ�������⣬��ֵ�����Ϊ6:2:2:1:1��д�����ַ���Ҫ���Y�Ľṹ��ʽ___________��__________��
��6��д���Լ�ȩ����ȩ���Ҷ���Ϊ��Ҫԭ�Ϻϳ����������۾��߷��Ӳ��ϡ��ۼ���ϩ���������� ���ĺϳ�·�ߣ����Լ���ѡ����_________________��
���ĺϳ�·�ߣ����Լ���ѡ����_________________��
���𰸡� ��ȩ ���۷�Ӧ 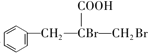
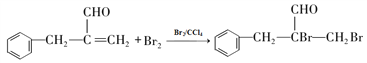
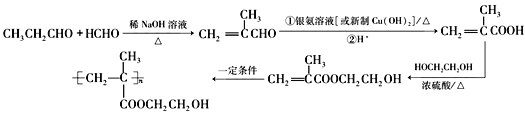
��������������Ϣ��AΪ����ȩ��ͬϵ���������������Է�������Ϊ134��A����ȩ������F��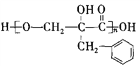 �Ľṹ��ʽ��֪��EΪ�����ǻ��ķ������ᣬ����C�ķ���ʽ������D��E������֪��C��DΪ�ǻ���ȩ����������D��EΪ±��ԭ�ӵ�ˮ�ⷴӦ�����E�Ľṹ��֪��DΪ������ԭ�ӵķ������ᣬ��CΪ������ԭ�ӵķ���ȩ��BΪ����̼̼˫���ķ���ȩ��������Ϣ�ڿ�֪XΪ��ȩ��
�Ľṹ��ʽ��֪��EΪ�����ǻ��ķ������ᣬ����C�ķ���ʽ������D��E������֪��C��DΪ�ǻ���ȩ����������D��EΪ±��ԭ�ӵ�ˮ�ⷴӦ�����E�Ľṹ��֪��DΪ������ԭ�ӵķ������ᣬ��CΪ������ԭ�ӵķ���ȩ��BΪ����̼̼˫���ķ���ȩ��������Ϣ�ڿ�֪XΪ��ȩ��
��1������������, XΪ��ȩ����ȷ�𰸣���ȩ��
��2������F�� ���ṹ��֪�������������ǻ����Ȼ��������۷�Ӧ���ɵģ���˸÷�Ӧ����Ϊ���۷�Ӧ����ȷ�𰸣����۷�Ӧ��
���ṹ��֪�������������ǻ����Ȼ��������۷�Ӧ���ɵģ���˸÷�Ӧ����Ϊ���۷�Ӧ����ȷ�𰸣����۷�Ӧ��
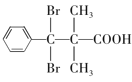
��3������C�ķ���ʽ������D��E������֪��C��DΪ�ǻ���ȩ����������D��EΪ±��ԭ�ӵ�ˮ�ⷴӦ�����E�Ľṹ��֪��DΪ������ԭ�ӵķ������ᣬD�Ľṹ��ʽΪ ����ȷ�𰸣�
����ȷ�𰸣� ��
��
��4�����������֪BΪ����̼̼˫���ķ���ȩ���������巢���ӳɷ�Ӧ����ѧ����ʽΪ��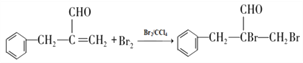 ����ȷ�𰸣�
����ȷ�𰸣�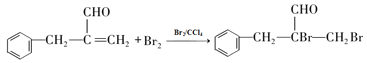 ��
��
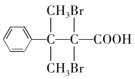
��5��D�Ľṹ��ʽΪ �������廯����Y��D��ͬϵ�˵���������Ȼ�����ԭ�ӣ��ܹ��뱥��Na2CO3��Һ��Ӧ�ų����壬˵�������Ȼ���������ֻ��1���������ṹ�ı仯ֻ��̼���칹����ԭ��λ���칹���˴Ź���������ʾ��5�ֲ�ͬ��ѧ�������⣬��ֵ�����Ϊ6:2:2:1:1��˵����������3����ԭ�ӣ�������Ϊ2:2:1����������2����ԭ�ӣ�������Ϊ6:1������Ҫ���Y�Ľṹ��ʽΪ
�������廯����Y��D��ͬϵ�˵���������Ȼ�����ԭ�ӣ��ܹ��뱥��Na2CO3��Һ��Ӧ�ų����壬˵�������Ȼ���������ֻ��1���������ṹ�ı仯ֻ��̼���칹����ԭ��λ���칹���˴Ź���������ʾ��5�ֲ�ͬ��ѧ�������⣬��ֵ�����Ϊ6:2:2:1:1��˵����������3����ԭ�ӣ�������Ϊ2:2:1����������2����ԭ�ӣ�������Ϊ6:1������Ҫ���Y�Ľṹ��ʽΪ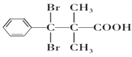 ��
��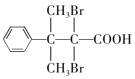 ����ȷ�𰸣�
����ȷ�𰸣�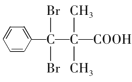 ��
�� ��
��
��6��Ҫ�ϳ��л���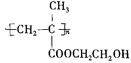 ���͵��Ⱥϳ�CH2=C(CH3)COOCH2CH2OH����Ҫ�ϳ�CH2=C(CH3)COOCH2CH2OH���͵��ü���ϩ�����Ҷ�������������Ӧ��������ϩ��͵��ɼ���ϩȩ��������������ȩ�ͼ�ȩ�ڼ��Ի����·�Ӧ���ɼ���ϩȩ������Ʊ������ʵ��������£�
���͵��Ⱥϳ�CH2=C(CH3)COOCH2CH2OH����Ҫ�ϳ�CH2=C(CH3)COOCH2CH2OH���͵��ü���ϩ�����Ҷ�������������Ӧ��������ϩ��͵��ɼ���ϩȩ��������������ȩ�ͼ�ȩ�ڼ��Ի����·�Ӧ���ɼ���ϩȩ������Ʊ������ʵ��������£�
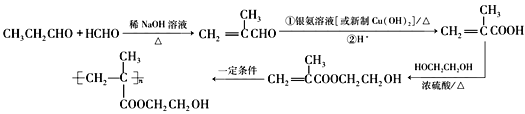 ����ȷ�𰸣�
����ȷ�𰸣�


 �����ܾ�ϵ�д�
�����ܾ�ϵ�д� ���ƿ�����ϵ�д�
���ƿ�����ϵ�д� ���¿쳵����������ϵ�д�
���¿쳵����������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��SO2��Σ����Ϊ���صĴ�����Ⱦ��֮һ��SO2�ĺ����Ǻ���������Ⱦ��һ����Ҫָ�ꡣ��ҵ�ϳ����ô���ԭ�������շ�����SO2������ԭSO2������������SO2��Ⱦ�����ҿɵõ��м�ֵ�ĵ���S��
��1���ڸ�����ִ��������£�CH4��ʹSO2ת��ΪS��ͬʱ����CO2 ��H2O����֪CH4��S��ȼ���ȷֱ�Ϊ890.3kJ/mol��297.2kJ/mol��CH4��SO2��Ӧ���Ȼ�ѧ����ʽΪ________________��
��2����H2��ԭSO2����S�ķ�Ӧ��������ɣ���ͼ1��ʾ���ù�����������ʵ����ʵ���Ũ����ʱ��ı仯��ϵ��ͼ2��ʾ��
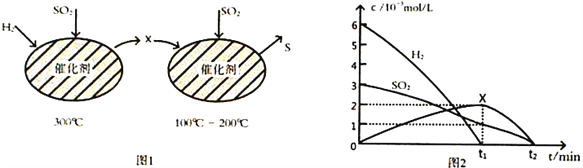
�ٷ�����֪XΪ____________(д��ѧʽ)��0��t1ʱ��ε��¶�Ϊ_________________��0��t1ʱ�����SO2 ��ʾ�Ļ�ѧ��Ӧ����Ϊ________________________________��
���ܷ�Ӧ�Ļ�ѧ����ʽΪ_______________________________��
��3����̿����ԭSO2����S2����ѧ����ʽΪ��2C(s)+2SO2(g)![]() S2(g)+2CO2(g)�����������У�lmol/LSO2�������Ľ�̿��Ӧ��SO2��ת�������¶ȵı仯��ͼ3��ʾ��
S2(g)+2CO2(g)�����������У�lmol/LSO2�������Ľ�̿��Ӧ��SO2��ת�������¶ȵı仯��ͼ3��ʾ��
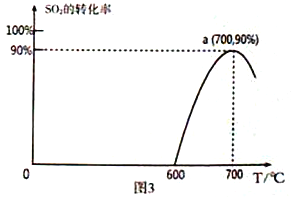
�ٸ÷�Ӧ�Ħ�H_______________0 ���������������
�ڼ���a���ƽ�ⳣ��Ϊ_______________________��
��4����ҵ�Ͽ���Na2SO3��Һ����SO2���÷�Ӧ�����ӷ���ʽΪ__________________________��25��ʱ��1mol/L��Na2SO3��Һ����SO2������ҺpH=7ʱ����Һ�и�����Ũ�ȵĴ�С��ϵΪ__________________����֪��H2SO3�ĵ��볣��K1=1.3��10-2��K2=6.2��10-8
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��������������������ɵ��ǣ� ��
A.���������Ũ������
B.ͭ�����ȵ�Ũ������
C.���������ϡ����
D.п�������ϡ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����������ˮ����ʢ��KI��Һ���Թ��У�����������CCl4 �� Ȼ�������ã������������ǣ� ��
A.��Һ�ֲ㣬�ϲ㼸����ɫ���²��Ϻ�ɫ
B.��Һ�ֲ㣬�ϲ��Ϻ�ɫ���ϲ㼸����ɫ
C.��Һ�ֲ㣬�ϲ㼸����ɫ���²�Ⱥ�ɫ
D.��Һ���Ϻ�ɫ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���������NH4Al(SO4)2��12H2O���Ƿ�����ѧ���õĻ��Լ�����ҵ�ϳ�����������Ҫ�ɷ�ΪAl2O3�������������������Z��ˮ��Һ�������˿��������乤������ͼ���£�
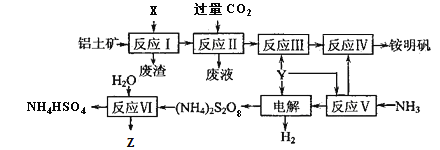
��1��д����Ӧ������ӷ���ʽ____________________��
��2��25��ʱ����0.2 mol��L-1�İ�ˮ��0.1 mol��L-1��Y��Һ�������ϣ�������Һ��pH=5������¶��°�ˮ�ĵ��볣��Kb��_____________�����Ի��ʱ��Һ����ı仯����
��3���ӷ�Ӧ�����������Һ�л������������ʵ���������Ϊ_____________��____________�����ˡ�ϴ�ӣ���������ƣ���
��4������⡱ʱ���ö��Բ������缫���������缫��ӦʽΪ__________________________��
��5����Ӧ���Ļ�ѧ����ʽΪ______________________��
��6����ˮ�к���Fe2����Mn2+�Ƚ������Ӷ���Ȼ���������ص��ƻ����ã�������(NH4)2S2O8������ȥ��д��Mn2+����������MnO2�����ӷ���ʽΪ__________________________________��ZҲ�н�ǿ�����ԣ���ʵ�������в���Z����Mn2+��ԭ����_______________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����й����л�ѧ��û�б��ƻ����ǣ� ��
A.ˮ���½��
B.ˮ���õ�����������
C.NaCl�����ۻ�
D.NaOH����ˮ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����1���������з�Ӧ��2SO2(g) + O2(g)![]() 2SO3(g) �����2min��SO2��Ũ����6 mol/L�½�Ϊ2 mol/L����SO2Ũ�ȱ仯����ʾ�Ļ�ѧ��Ӧ����Ϊ_________����O2Ũ�ȱ仯����ʾ�ķ�Ӧ����Ϊ_________�������ʼʱSO2Ũ��Ϊ4mol/L��2min��Ӧ��ƽ�⣬�����ʱ����v(O2)Ϊ0.5mol/(L��min)����ô2minʱSO2��Ũ��Ϊ_________��
2SO3(g) �����2min��SO2��Ũ����6 mol/L�½�Ϊ2 mol/L����SO2Ũ�ȱ仯����ʾ�Ļ�ѧ��Ӧ����Ϊ_________����O2Ũ�ȱ仯����ʾ�ķ�Ӧ����Ϊ_________�������ʼʱSO2Ũ��Ϊ4mol/L��2min��Ӧ��ƽ�⣬�����ʱ����v(O2)Ϊ0.5mol/(L��min)����ô2minʱSO2��Ũ��Ϊ_________��
��2����ͼ��ʾ���ܱ������з�Ӧ��2SO2+O2![]() 2SO3 ��H��0�ﵽƽ��ʱ�����������ı������Ӧ�ٶȺͻ�ѧƽ��ı仯�����a~b�����иı������������________��b~c�����иı������������_________��
2SO3 ��H��0�ﵽƽ��ʱ�����������ı������Ӧ�ٶȺͻ�ѧƽ��ı仯�����a~b�����иı������������________��b~c�����иı������������_________��

��3�����Ϸ�Ӧ��ƽ�������ʱֻ���������������Ϊԭ����2��������ƽ��ʱ���������¶Ƚ�_______�����������������Ƚ���,������������������������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ͼ��ʾϸ����ijЩ�л����Ԫ����ɺ��ܹ�ϵ������A��B����Ԫ�أ����������������ӣ�ͼ��X��Y��Z��P�ֱ�Ϊ�����������ӵĻ�����λ����ش��������⡣

��1����������л������ͨ����ɫ��Ӧ���������������ʹ��___________Ⱦɫ�����γ���________��________��________�����յ���ȾҺ����������________��ʹ��________������ʱ����_________________��ҡ�Ⱥ����________��
��2����ɢ�Ļ�����λ����________�֣��������______�֡�
��3�����Ľṹ���ж����Ե�ֱ��ԭ����_______________________________________��
��4����С��������X��Y��Z��P���ɴ�������ʢ��Ĺ����У���һ��ͬ����������________��
��5����ͬ�����Ģ������ֽ��ʱ�ͷŵ�����ԶԶ���ڢ�ԭ���Ǣ���________�����ߡ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����������м��ܸ�ϡH2SO4��Ӧ�����ܸ�����������Һ��Ӧ���ǣ� �� ��NaHCO3��Al2O3��Al��OH��3 ��Al��
A.�ۢ�
B.�ڢۢ�
C.�٢ۢ�
D.ȫ��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com