
����Ŀ��������������������ͷ�չ����Ҫ���ʻ������ش��������⣺
(1)�������������ܻ�ȡ�������ǽ������ϵ��� ______ (�����)��
�ٲ��ü���Ϊԭ�ϣ������������������������ʯ
�ڲ��ô�ͳ�����ý�̿��ԭSiO2�Ʊ�������
����ˮ�����������м����¯��������������;��ˮ��
���ڲ������������м���K��Pb��������������;�Ĺ�ѧ����
(2)ij������¯��һ��Ͷ�������ұ�����������ù�����ת�Ƶĵ���Ϊ1.60��105mol�������������ķ�Ӧ��Ҳ�����������е����ʣ������õ�����Ϊ ______t�������к�̼�����Ԫ�ؽ϶࣬������������Ϊԭ�ϣ���ȥ�����̼���������Ԫ�أ����г�ȥ��Ļ�ѧ����ʽΪ ______��
(3)��ҵ������Al�Ĺ����������£�
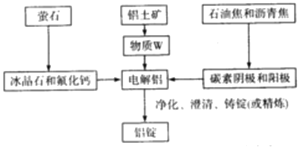
�ù����б���ʯ�ͷ����Ƶ������� ______������W�Ļ�ѧʽΪ ______ ����������Ĵ���Ϊ60%����ȡW����ʧ��Ϊ3%�����ʱ��ʧ��Ϊ0.1%����������ʱ����ʧ��Ϊ2%�����������ĺ���Ϊ99.9%����ô1.0t��������Ʊ����� ______t��
(4)������ʴ��������ʧ��������������ʴ��Ϊ������ʴ����������ӦʽΪ ______������������ʩ�ܶ࣬������������(����Zn)������������������������ӦʽΪ ______��
���𰸡��٢� 3.36 FeS+CaO![]() FeO+CaS ����Al2O3���۵㣬�������ڵ���ʵ��ܺ� Al2O3 0.3 O2+2H2O+4e-=4OH- Zn-2e-=Zn2+
FeO+CaS ����Al2O3���۵㣬�������ڵ���ʵ��ܺ� Al2O3 0.3 O2+2H2O+4e-=4OH- Zn-2e-=Zn2+
��������
(1)��ͳ���ǽ��������ǹ�ҵ�ͻ�������������Ļ������ϣ��粣����ˮ��ȣ��������ǽ���������20���������Ժ�չ�����ģ������������ܺ���;�IJ��ϣ��������ִ��¼������²�ҵ����ͳ��ҵ�������졢�ִ�����������ҽѧ������ȱ�ٵ����ʻ������ݴ˽����жϣ�
(2)���������Ҫ�ɷ�Ϊ���������������ݵ����غ��ȼ���������������ʵ������ټ��������������������������д���������������Ʒ�Ӧ�Ļ�ѧ����ʽ��
(3)����ʯ���ܽ�Al2O3���壬���������ۼ�����Al2O3���۵㣻WΪ���������Ҫ�ɷ����������������������������Ȼ��������Ԫ�������غ���ʽ���㣻
(4)��������(����Zn)����������������������пʧȥ��������п���ӣ��ݴ�д���缫��Ӧʽ��
(1)�ٲ��ü���Ϊԭ�ϣ����������������������Ľ��ʯΪ�������ǽ������ϣ�����ȷ��
�ڲ��ô�ͳ�����ý�̿��ԭSiO2�Ʊ��ĵ�����Ϊ�������ǽ������ϣ�����ȷ��
��ˮ���Ǵ�ͳ���ǽ������ϣ��۴���
�ܹ�ѧ�����Ǵ�ͳ���ǽ������ϣ��ܴ���
�ʺ���ѡ���Ǣ٢ڣ�
(2)������������ѧʽΪFe3O4��������Ԫ�ص�ƽ�����ϼ�Ϊ+![]() ���ù�����ת�Ƶĵ���Ϊ1.60��105mol��������Fe�����ʵ���n(Fe)=
���ù�����ת�Ƶĵ���Ϊ1.60��105mol��������Fe�����ʵ���n(Fe)=  =6��104mol�����Եõ�����������Ϊ��m=56g/mol��6��104mol=3.36��106g=3.36t��ͨ����CaO��ȥ��Ӧ��ѧ����ʽΪ��FeS+CaO
=6��104mol�����Եõ�����������Ϊ��m=56g/mol��6��104mol=3.36��106g=3.36t��ͨ����CaO��ȥ��Ӧ��ѧ����ʽΪ��FeS+CaO![]() FeO+CaS��
FeO+CaS��
(3)Al2O3�������Ӿ��壬�۵�ϸߣ��������ʯ�ͷ����ƣ����ڵı���ʯ�ͷ��������ܽ�Al2O3���壬�������ڵ��ұ����ʱ���������ۼ������������������ۻ�ʱ������¶ȣ��ڽ�������ұ���м������ʯ�ͷ����Ƶ�Ŀ���ǽ���Al2O3���۵㣬�������ڵ���ʵ��ܺģ�W������������Ҫ�ɷ�����������ѧʽΪAl2O3��
�����ɵ�����������Ϊx��������Ԫ�������غ�ɵã�1.0t��60%��![]() ��(1-3%)��(1-0.1%)��(1-2%)=99.9%x����ã�x=0.3t��
��(1-3%)��(1-0.1%)��(1-2%)=99.9%x����ã�x=0.3t��
(4)��������ʴ�У������������õ��ӷ�����ԭ��Ӧ���缫��ӦʽΪ��O2+2H2O+4e-=4OH-���ڽ���������ʩ�У���������(����Zn)������Ϊԭ��ص�������������ԭ��Ӧ����������(����Zn)Ϊ������Znʧȥ��������Zn2+���缫��ӦʽΪ��Zn-2e-=Zn2+��


 �㽭��У��ʦ���ϵ�д�
�㽭��У��ʦ���ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ij��ѧѧϰС���������ʵ�鷽�����ⶨij����NaCl��С�մ���Ʒ��![]() ������������
������������
������һ�������ϣ�NaCl������![]() ʱ�ۻ������ֽ⣬
ʱ�ۻ������ֽ⣬![]() ���ȷֽ⣬
���ȷֽ⣬![]() ���ɴ����ʵ�飺�õ�����ƽ��ȡ
���ɴ����ʵ�飺�õ�����ƽ��ȡ![]() ��Ʒ�������������þƾ��Ƽ��ȣ���ͼ�������¶ȸ���
��Ʒ�������������þƾ��Ƽ��ȣ���ͼ�������¶ȸ���![]() ��������
��������![]() ���������غ���ȴ������ʣ���������Ϊ
���������غ���ȴ������ʣ���������Ϊ![]() ��
��
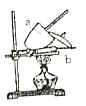
��1��ʵ�������ٳ���____�Ρ�
��2��ʵ���У��ﵽ���ز����ı���__________��
����������������![]() ��Һ�ʼ��ԣ�
��Һ�ʼ��ԣ�![]() �����������ʵ�飺ȷ��ȡ1.000 g��Ʒ��������ƿ���100 mL��Һ���õζ�����ȡ20.00 mL����ƿ�У�����2�μ���Ϊָʾ������0.1000mol/L�������Һ�ζ���ƽ�����ݣ�����ʵ����������£�
�����������ʵ�飺ȷ��ȡ1.000 g��Ʒ��������ƿ���100 mL��Һ���õζ�����ȡ20.00 mL����ƿ�У�����2�μ���Ϊָʾ������0.1000mol/L�������Һ�ζ���ƽ�����ݣ�����ʵ����������£�
1 | 2 | |
| 20.00 | 20.00 |
| 0.00 | 0.20 |
| 19.98 | 20.22 |
��3��ʵ���У���������������ȷʱ�����в�����������ʵ����������______����
A������ƿ������ˮϴ����ƿ����ˮ������ֱ��������Һ
B���ζ����ڱ���ˮ���װ���Һ
C����ƿ�ڱ���ˮ�飬�ô���Һ��ϴ����ʹ��
D����ƿ������ˮϴ����ֱ�ӷ������Һ���вⶨ
span>��4���ζ��յ���жϣ�__________��
��5������ʵ���������������ƽ��ֵΪ__________mL��
��6����Ʒ��![]() ����������__________��
����������__________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ij�����ܱ������У������ʵ���֮��Ϊ 1:1 Ͷ�� A �� B���������·�Ӧ��A(g)+ B(��)![]() aD(g)(a Ϊ������)����ƽ�����������¶�ʱ��D �������������ӣ���Сѹǿ��D ������������С����һ���¶Ⱥ�һ��ѹǿ�´�ƽ��ʱ��D �����ʵ�������Ϊ 50%��������˵����ȷ����
aD(g)(a Ϊ������)����ƽ�����������¶�ʱ��D �������������ӣ���Сѹǿ��D ������������С����һ���¶Ⱥ�һ��ѹǿ�´�ƽ��ʱ��D �����ʵ�������Ϊ 50%��������˵����ȷ����
A.B �����ǹ���Ҳ������Һ��
B.�÷�Ӧ�ġ�H��0
C.��һ���¶Ⱥ�һ��ѹǿ�´�ƽ��ʱ��A ��ת����Ϊ 67%
D.����������ܶȲ���ʱ���÷�Ӧ��ƽ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�������½�0.1mol�������ʷֱ�Ͷ��90gˮ�г�ֻ�ϣ���Na��NaOH��Na2O��Na2O2��������Һ��OH-���ʵ���Ũ�ȴ�С����˳����ȷ���ǣ� ��
A.��>��>��>��B.��>��>��>��
C.��>��=��>��D.��=��>��>��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��������������ͭ�Ͻ���Ʒ![]() ��������Ϊ
��������Ϊ![]() ��
��![]() ��
��![]() �������´���������˵����ȷ����
�������´���������˵����ȷ����![]()
![]()
A.��ҺA�е�������Ϊ![]() ��
��![]() ��
��![]()
B.��Ʒ��FeԪ�ص�����Ϊ![]()
C.��Ʒ��CuO������Ϊ![]()
D.![]()
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�������йؾ���������У�����ȷ����(����)
A.���ʯ��״�ṹ�У��ɹ��ۼ��γɵ�̼ԭ�ӻ��У���С�Ļ�����6��̼ԭ��
B.�Ȼ��ƾ����У�ÿ��Na+��Χ��������ҽ��ڵ�Na+����6��
C.�Ȼ�菉����У�ÿ��Cs+��Χ����8��Cl-
D.�ɱ������У�ÿ��CO2������Χ����12��CO2����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���йؾ���Ľṹ����ͼ��ʾ������˵��������ȷ����
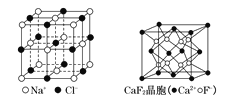
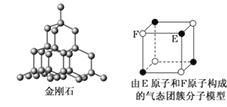
A.��NaCl�����У���Na�������Cl���γ���������
B.��CaF2�����У�ÿ������ƽ��ռ��4��Ca2��
C.�ڽ��ʯ�����У�6��̼ԭ���γ�һ�����Ҳ���ͬһƽ����
D.����̬�Ŵط��ӵķ���ʽΪEF��FE
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ij�о�С��Ϊ��̽��һ��dz��ɫ��X(��������Ԫ�أ������ᾧˮ��M(X)<908gmol-1)����ɺ����ʣ���Ʋ����������ʵ�飺
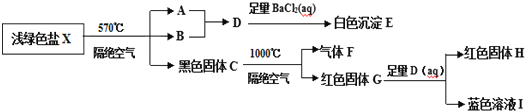
ȡһ������dz��ɫ��X��������ʵ�飬��ַ�Ӧ��õ�23.3g��ɫ����E��28.8g��ɫ����G��12.8g��ɫ����H��
��֪����dz��ɫ��X��570�桢�����������������ȷֽ�Ϊ��������ԭ��Ӧ��
�ڳ�����B��Һ̬��1��B���Ӻ���10�����ӡ�
��ش��������⣺
��1��д��B���ӵĵ���ʽ___��
��2����֪G����ϡ���ᣬ��Һ�����ɫ�����ų���ɫ���塣��д���÷�Ӧ�����ӷ���ʽΪ___��
��3���ڸ���������570���¶��¼���X����ȫ�ֽ�Ļ�ѧ��Ӧ����ʽΪ___��
��4��һ�������£�NH3���ɫ����C����������ԭ��Ӧ�õ���ɫ����������(���Ǵ�����Ҫ�ɷ�֮һ)��д��һ�����ܵĻ�ѧ��Ӧ����ʽ___��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���������Ƴ�����Ư����ij��ѧС�����ʵ���Ʊ��������ƣ����������ʷ�����
ʵ��I ��ͼװ���Ʊ�NaC1O2����

��֪����C1O2Ϊ����ɫ���壬������ˮ��Ӧ��
��NaC1O2������Һ�ڵ���38��ʱ����NaC1O2��3H2O���壬����38��ʱ����NaC1O2���壬�¶ȸ���60��ʱNaC1O2�ֽ�����NaC1O3��NaCl��
��1��װ��A��b������������____��a���ܷ���ϡ�������Ũ����____������������������������ԭ����____��
��2��A������C1O2�Ļ�ѧ��Ӧ����ʽΪ____��
��3��C������NaC1O2ʱH2O2��������____��Ϊ��ø����NaC1O2������C������װ�ý��иĽ�����ʩΪ____��
��4����Ӧ�����в���ɴ�Cװ�õ���Һ�л��NaC1O2���壬�벹�����ơ�
i.55��ʱ�����ᾧ ii.__________ ii.��40����ˮϴ�� iv.����60�����õ���Ʒ
ʵ��� ��Ʒ���ʷ���
��5������ʵ�����Ƶõ�NaC1O2�����л����ܺ���������ѧ�����ĺ������Σ��仯ѧʽΪ____��ʵ���пɼ��ٸ����ʲ����IJ�����������____��дһ������
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com