
����A��B��C��D����ǰ������Ԫ����ɵij�����ͬ���ʻ������֮��������ת����ϵ��

��1����A��B��C��D�����������AΪ���ά����Ҫ���ϣ���д������ѧ��Ӧ����ʽ�� _______???????????? __________
��2����A��B��C��D������ͬһ�ֳ�������Ԫ�أ�B�Ǻ�ɫ���Թ��壬D��һ�ֺ��ɫ����,��Ӧ���ڳ���ϡ���н��У�C�Ǹ÷�Ӧ�����ɵ�Ψһ�Σ���Ӧ�������ӷ���ʽ��________ _______, C��ˮ��ҺPH______7 (����>����<���� =��)��
��3����A��B��C��D������ͬһ�ֶ����ڵĽ���Ԫ��,��B��D����Һ�з�Ӧ���ɳ���C,�������ж���ȷ����______(����Сд����ĸ)��
a��A������һ���ͻ���ϣ�
b��B����Һһ���ʼ��ԣ�
c��C һ�����������������ڿ�������Һ��?
��AΪ�������ʣ�����1molA�����Ʊ�C,������_____mol HCl��_____mol NaOH��
��.������ȫ�������г���ȫ����Ҫ���ϡ�������������ײ��˲�䣬��ȫװ��ͨ����ʹ���еĹ����ĩ�ͷų������ĵ����γ����ң��Ӷ�����˾�����˿������˺���Ϊ�о���ȫ���ҹ����Ļ�ѧԭ����ȡ��ȫװ���еĹ����ĩ����ʵ�顣����ɷ�����ȷ���÷�ĩ������Na��Fe��N��O����Ԫ�ء�ˮ����ʵ������������ĩ�����ܽ⡣����⣬������Ϊ������ף�������Ϊ����ɫ���壬���������ᡣȡ13.0g������ף�Ħ������Ϊ65g/ mol��������ʹ����ȫ�ֽ⣬���ɵ����͵����ң����ɵĵ����ۺϳɱ�״���µ����Ϊ6.72L���������ڸ��¸����������������벻�������ɫ��ĩ��Ӧ����һ�ּ��������������һ�ֵ��ʡ��������������Ӵ���ת��Ϊ�������Ρ�
��ش��������⣺
��1�������ȷֽ�Ļ�ѧ����ʽΪ??????????????????? ��
��2�����ĵ���ʽΪ??????????? ��
��3�����������У��п�����Ϊ��ȫ�����к���ɫ��ĩ���Ʒ����??????????? ��
A��KCl??????????? B��KOH?????????? C��Na2S????????????? D��CuO
������1��2Mg+CO2 2MgO+C(2��)
2MgO+C(2��)
��2��3Fe3O4+28H++NO3-=9Fe3++NO��+14H2O(2��)?????? <?? (2��)
��3��ac? (2��) ? 0.75(2��) ? 0.75(2��)
������1��2NaN3=2Na+3N2��? (2��)????????? ��2��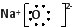 (2��)????? ��3��D (2��)
(2��)????? ��3��D (2��)
��������
�����������1����A��B��C��D�����������AΪ�������裬��϶�����������ʣ�BΪCO��CΪCO2��DΪMgO������ѧ��Ӧ����ʽΪ��2Mg+CO2 2MgO+C����2��B�Ǻ�ɫ���Թ���ΪFe3O4��D��һ�ֺ��ɫ����Ϊ����������C�Ǹ÷�Ӧ�����ɵ�Ψһ�Σ�������Ϊ���ᣬ��Ӧ�������ӷ���ʽ��3Fe3O4+28H++NO3-=9Fe3++NO��+14H2O��CΪ��������ˮ��Һˮ�������ԡ���3�������ڽ���Ԫ���У�����������ֻ�������仯���A��������������BΪ�����ӻ�ƫ�������CΪ����������DΪƫ������������ӡ��������۵�ߣ������ͻ���ϣ��������������ԣ������������������ڿ�������Һ��
2MgO+C����2��B�Ǻ�ɫ���Թ���ΪFe3O4��D��һ�ֺ��ɫ����Ϊ����������C�Ǹ÷�Ӧ�����ɵ�Ψһ�Σ�������Ϊ���ᣬ��Ӧ�������ӷ���ʽ��3Fe3O4+28H++NO3-=9Fe3++NO��+14H2O��CΪ��������ˮ��Һˮ�������ԡ���3�������ڽ���Ԫ���У�����������ֻ�������仯���A��������������BΪ�����ӻ�ƫ�������CΪ����������DΪƫ������������ӡ��������۵�ߣ������ͻ���ϣ��������������ԣ������������������ڿ�������Һ��
��.������Ŀ������Ϣ�����������Թ���Ϊ������������0.2Ħ���ֽ�õ�����0.3Ħ�����������Ԫ�ؿ�֪��ΪNaN3�������ȷֽ�Ļ�ѧ����ʽΪ��2NaN3=2Na+3N2�����Ƶ�������������ֻ���������Ǽ�����������Ա�Ϊ�����ƣ�����ʽΪ��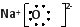
���㣺����Ԫ�ؼ��仯������ƶϡ���ѧ����ʽ������ʽ����д��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ||
| ||
| ||
| ||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
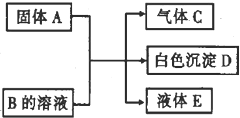 ��2012?��ɫ��ģ��A��B��C��D��Ϊ��ѧ��ѧ�е����ʣ����Ǽ�ķ�Ӧ��ϵ����ͼ��ʾ��
��2012?��ɫ��ģ��A��B��C��D��Ϊ��ѧ��ѧ�е����ʣ����Ǽ�ķ�Ӧ��ϵ����ͼ��ʾ��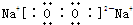
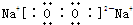
| 7 |
| 2 |
| 7 |
| 2 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ��֪A��B��C��D��Ϊ���壬E��F��Ϊ�����³ʹ�������ӻ����GΪ�Ȼ��ƣ�A��B��ȼ�յĻ���ʲ�ɫ����Ӧ���������������д������̣�����֮���ת����ϵ��ͼ��ʾ��
��֪A��B��C��D��Ϊ���壬E��F��Ϊ�����³ʹ�������ӻ����GΪ�Ȼ��ƣ�A��B��ȼ�յĻ���ʲ�ɫ����Ӧ���������������д������̣�����֮���ת����ϵ��ͼ��ʾ��

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
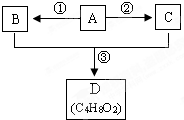 A��B��C��D��Ϊ�������л����һ�������£���������ͼ��ʾ��ת����ϵ��
A��B��C��D��Ϊ�������л����һ�������£���������ͼ��ʾ��ת����ϵ��| ���� |
| �� |
| ���� |
| �� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
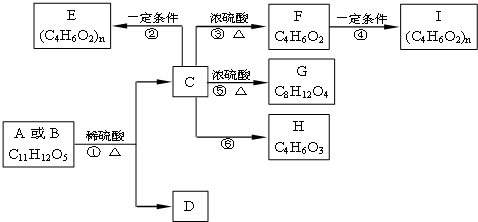
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com