
����Ŀ��A��B��C��DΪԭ�������������������Ԫ�أ�A2����B+������ͬ�ĵ��ӹ��ͣ�C�� DΪͬ����Ԫ����C�������������������������3����DԪ���������һ��δ�ɶԵ��ӡ��ش��������⣺
��1������Ԫ���е縺����С����________����Ԫ�ط��ţ�������Cԭ�ӵ���Χ�����Ų�ͼΪ________��
��2��A��B���⻯�������ľ������ͷֱ�Ϊ_________��_________��
��3��B��C��������D�γɻ���������۵�ϸߵ���____���û�ѧʽ��ʾ��
��4��A��B���γ�1:1�͵Ļ�����E��E�ĵ���ʽΪ_____
��5��������D2A�����幹��Ϊ_________������ԭ�ӵŵ��Ӷ���Ϊ_________������D��ʪ���Na2CO3��Ӧ���Ʊ�D2A���仯ѧ����ʽΪ_______________��
��6��A��B�ܹ��γɻ�����F���侧���ṹ��ͼ��ʾ�������߳�Ϊ0.566nm�� F �Ļ�ѧʽΪ______��������A ԭ�ӵ���λ��Ϊ______������F���ܶ�=______g��cm��3��ֻ��ʽ�������㣩

���𰸡���1��Na��1�֣��� ��1�֣�![]()
��2�����Ӿ��壨1�֣� ���Ӿ��壨1�֣�
��3��NaCl
��4��![]()
��5��V��2��
2Cl2��2Na2CO3��H2O��Cl2O��2NaHCO3��2NaCl
��6��Na2O��8��![]() ����NA��ʾҲ�ɣ�
����NA��ʾҲ�ɣ�
�����������������C�������������������������3����ӦΪPԪ�أ�C��DΪͬ����Ԫ�أ���ӦΪ��������Ԫ�أ�DԪ���������һ��δ�ɶԵ��ӣ�ӦΪClԪ�أ�A2-��B+������ͬ�ĵ��ӹ��ͣ����ԭ��������ϵ��֪AΪOԪ�أ�BΪNaԪ�أ�
��1������Ԫ�طֱ�ΪO��Na��P��Cl���縺������ΪOԪ�أ�CΪPԪ�أ���������Ų�Ϊ1s22s22p63s23p3���۵����Ų�ͼΪ![]() ��
��
��2��A���⻯��Ϊˮ��Ϊ���Ӿ��壬B���⻯��ΪNaH��Ϊ���Ӿ��壬
�ʴ�Ϊ��O3��O3��Է��������ϴ��»����ϴ��Ӿ��壻���Ӿ��壻
��3��Na��P���ܺ�Cl�γɻ�����NaCl��PCl3������NaClΪ���Ӿ��壬�۷е�ϸߣ�
��4��O��Na���γ�1:1�͵Ļ�����Na2O2�������ʽΪ![]() ��
��
��5��������D2AΪCl2O��OΪ����ԭ�ӣ��γ�2���������µ��Ӷ���Ϊ![]() =2��������ԭ�ӵļ۲���Ӷ���Ϊ4�����幹��ΪV�Σ�������ʪ���Na2CO3��Ӧ���Ʊ�Cl2O����Ӧ�ķ���ʽΪ2Cl2+2Na2CO3+H2O=Cl2O+2NaHCO3+2NaCl��
=2��������ԭ�ӵļ۲���Ӷ���Ϊ4�����幹��ΪV�Σ�������ʪ���Na2CO3��Ӧ���Ʊ�Cl2O����Ӧ�ķ���ʽΪ2Cl2+2Na2CO3+H2O=Cl2O+2NaHCO3+2NaCl��
��6��A��B�ܹ��γɻ�����FΪ���ӻ����������λ�ھ����Ķ�������ģ�������λ�ھ��������ģ���Na�ĸ���Ϊ8��O�ĸ���Ϊ8��+6��=4��N��Na����N��O��=2��1�����γɵĻ�����ΪNa2O��������Oλ�ڶ��㣬Naλ�����ģ�ÿ����������1��Na��O�ľ��������ÿ������Ϊ8���������У�����Oԭ�ӵ���λ��Ϊ8������������Ϊ![]() �����������Ϊ��0.566��10-7��cm3������F���ܶ�Ϊ
�����������Ϊ��0.566��10-7��cm3������F���ܶ�Ϊ![]() ��
��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���������ڲ�ͬ�ܼ�����NaOH������ͬ���͵ķ�Ӧ�����ɲ�ͬ�ķ�Ӧ���ijͬѧ��������������ʣ�����ͼʵ��װ��(����̨���ƾ�����)��֤ȡ����Ӧ����ȥ��Ӧ�IJ������һ�����̽����
ʵ������������Թ��м���5 mL 1 mol/L NaOH��Һ��5 mL�����飬��
ʵ�����II�����Թ���ͼ�̶���ˮԡ���ȡ�

��1����ˮԡ���ȶ���ֱ���þƾ��Ƽ��ȵ�ԭ����________��
��2���۲쵽___________����ʱ��������������NaOH��Һ����ȫ��Ӧ��
��3���������������Ҵ��Ľṹ�����õIJ�����_____��
��4��Ϊ֤����������NaOH�Ҵ���Һ�з���������ȥ��Ӧ��������Ƶ�ʵ�鷽���У���Ҫ�������____������ķ����� (��˵�������õ��Լ�����ʵ�������Ԥ�������ʵ������)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����.ijʵ��С���H2O2�ķֽ���������̽�����±��Ǹ�ʵ��С���о�Ӱ��H2O2�ֽ����ʵ�����ʱ��¼��һ�����ݣ���������ͬ��״̬��ͬ��MnO2�ֱ����ʢ��15 ml 5%��H2O2��Һ�Ĵ��Թ��У����ô����ǵ�ľ�����ԣ�������£�

��1��д������ʵ���з�����Ӧ�Ļ�ѧ����ʽ ��
��2��ʵ���������������Ĵ�Ч���� �йء�
��. ���о�֪Cu2+��H2O2�ֽ�Ҳ���д����ã�Ϊ�Ƚ�Fe3+��Cu2+��H2O2�ֽ�Ĵ�Ч����ij�о�С���ͬѧ�ֱ��������ͼ�ס�����ʾ��ʵ�顣�ش�������⣺
��3�����Է�������ͼ��ͨ���۲� �����ԱȽϵó����ۡ���ͬѧ�����FeCl3��ΪFe2(SO4)3��Ϊ�������������� ��

��4��������������ͼ����ʾ��ʵ��ʱ��������40 mL����Ϊ����������Ӱ��ʵ������ؾ��Ѻ��ԡ�ͼ������A������Ϊ ��ʵ������Ҫ������������ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���л�������G�Ǻϳ�ά������ҩ����м��壬��ϳ�·�����£�

����A��F�ֱ����һ���л�������ϳ�·���в��ֲ��P��Ӧ��������ȥ��֪��

GΪ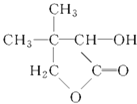 ��
��
��ش��������⣺
��1��G�ķ���ʽ_____________��D�й����ŵ�������_________��
��2����������Ӧ�Ļ�ѧ����ʽΪ__________________________��
��3����������Ӧ�Ļ�ѧ����ʽΪ__________________________��
��4��д��F�Ľṹ��ʽ_____________��
��5������~������Ӧ�����ڼӳɷ�Ӧ����___________________������ȡ����Ӧ����
__________________________�����������
��6��ͬʱ��������������E��ͬ���칹����_____________�֡�
��ֻ��һ�ֹ����ţ�
����״�ṹ���ޡ�O��O����
���˴Ź�������ֻ��2��塣
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����֪��ӦX+Y�TM+NΪ���ȷ�Ӧ���Ը÷�Ӧ��˵����ȷ��
A. X������һ������M
B. Y������һ������N
C. X��Y��������һ������M��N��������
D. ��Ϊ�÷�ӦΪ���ȷ�Ӧ���ʲ��ؼ��ȾͿɷ���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��������ͼװ�òⶨ�к��ȵ�ʵ�鲽�����£�

����ȡ50mL 0.25mol/L H2SO4��Һ����С�ձ��У������¶ȣ�
����ȡ50mL 0.55mol/L NaOH��Һ�������¶ȣ�
�۽�NaOH��Һ����С�ձ���,��Ͼ��Ⱥ�������Һ�¶ȡ���ش�
��1������ͼ��ʾ������A��������_________ ______��
��2��NaOH��Һ�Թ�����ԭ�� ____________________________��
��3������NaOH��Һ����ȷ������_______������ĸ����
A���ز������������� B��һ��Ѹ�ټ��� C�������μ���
��4��ʹ������NaOH��Һ��Ͼ��ȵ���ȷ������ ________ __________________��
��5������Һ���ܶȾ�Ϊ1g��cm-3,�кͺ���Һ�ı�����c=4.18 J����g���棩-1�������ʵ������д�����к��ȵ��Ȼ�ѧ����ʽ_______________________________

��6������ʵ����ֵ�����57.3 kJ/mol��ƫ�����ƫ���ԭ������ǣ�����ĸ��
a��ʵ��װ�ñ��¡�����Ч����
b���ֶ�ΰ�NaOH��Һ����ʢ�������С�ձ���
c�����¶ȼƲⶨNaOH��Һ��ʼ�¶Ⱥ�ֱ�ӲⶨH2SO4��Һ���¶�
��7����������ȷ����ȡ���Һ������¶ȣ�_______ ____________________
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�������й����ʵ����ʻ�Ӧ�õ�˵����ȷ����
A��������ǹ�����Ʒ����Ҫ��ѧ�ɷ� B���Ͻ����ٺ��������ϵĽ���Ԫ��
C������ɲ��������ЧӦ D��ʯ�ͷ���ɻ����ϩ������ͱ�ϩ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ⷨ����������������нϸߵĻ���Ч��;���Ч�棨ͼ�е缫��Ϊʯī����


��1�����NO�Ʊ�NH4NO3ԭ������ͼ��ʾ��
������Ϊ_______ (��X��Y)��Y�ĵ缫��ӦʽΪ________________________________��
��Ϊʹ��������ȫת��ΪNH4NO3����Ҫ���������A�Ļ�ѧʽΪ________________��
��2������ͼװ�ý���ģ����NO2����ʵ�飬�ɻ������ᡣ
�ٵ��ʱNO2������Ӧ�ĵ缫��Ӧʽ_________________________________��
�����б�״����2.24 LNO2�����գ�ͨ�������ӽ���Ĥ��ֻ����������ͨ������H+Ϊ_________________mol��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����Ǻ��ա��������������������Ҫԭ�ϡ���ҵ��������������FeTiO3��ұ������Ti���Ĺ����ǣ���2FeTiO3��6C��7Cl2![]() 2TiCl4��2FeCl3��6CO
2TiCl4��2FeCl3��6CO
�������������2Mg��TiCl4![]() Ti��2MgCl2
Ti��2MgCl2
�����жϲ���ȷ������ ��
A����Ӧ�������û���Ӧ
B����Ӧ�����Ȼ�������������ֲ���
C����Ӧ�١�������Ԫ�صĻ��ϼ۶��ı�
D����Ӧ���У����ֻ��Ϊ�����������μӷ�Ӧ
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com