
ЁОЬтФПЁПгаЛњЛЏКЯЮяGЪЧКЯГЩЮЌЩњЫиРрвЉЮяЕФжаМфЬхЃЌЦфКЯГЩТЗЯпШчЯТЃК

ЦфжаAЁЋFЗжБ№ДњБэвЛжжгаЛњЛЏКЯЮяЃЌКЯГЩТЗЯпжаВПЗжВњЮяМАЗДгІЬѕМўвбТдШЅвбжЊЃК

GЮЊ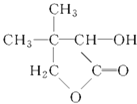 ЃЛ
ЃЛ
ЧыЛиД№ЯТСаЮЪЬтЃК
ЃЈ1ЃЉGЕФЗжзгЪН_____________ЃЛDжаЙйФмЭХЕФУћГЦЪЧ_________ЁЃ
ЃЈ2ЃЉЕкЂкВНЗДгІЕФЛЏбЇЗНГЬЪНЮЊ__________________________ЁЃ
ЃЈ3ЃЉЕкЂлВНЗДгІЕФЛЏбЇЗНГЬЪНЮЊ__________________________ЁЃ
ЃЈ4ЃЉаДГіFЕФНсЙЙМђЪН_____________ЁЃ
ЃЈ5ЃЉЕкЂй~ЂоВНЗДгІжаЪєгкМгГЩЗДгІЕФга___________________ЃЛЪєгкШЁДњЗДгІЕФга
__________________________ЁЃЃЈЬюВНжшБрКХЃЉ
ЃЈ6ЃЉЭЌЪБТњзуЯТСаЬѕМўЕФEЕФЭЌЗжвьЙЙЬхга_____________жжЁЃ
ЂйжЛКЌвЛжжЙйФмЭХЃЛ
ЂкСДзДНсЙЙЧвЮоЁЊOЁЊOЁЊЃЛ
ЂлКЫДХЙВеёЧтЦзжЛга2зщЗхЁЃ
ЁОД№АИЁПЃЈ1ЃЉC6H10O3ѕЅЛљЁЂШЉЛљЁЂєЧЛљЖраДЁЂТЉаДЁЂаДДэОљВЛЕУЗж
ЃЈ2ЃЉ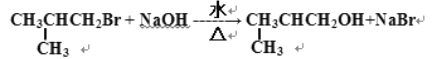
ЃЈ3ЃЉ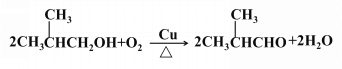
ЃЈ4ЃЉ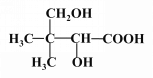
ЃЈ5ЃЉЂйЂмЂоЁЂЂкЂн ЃЈТЉаДЕУвЛЗжЃЌаДДэВЛЕУЗжЃЉ ЃЈ6ЃЉ3
ЁОНтЮіЁП
ЪдЬтЗжЮіЃКвьЖЁЯЉКЭфхЛЏЧтЗЂЩњМгГЩЗДгІЩњГЩфхДњЬўAЃЌAКЭЧтбѕЛЏФЦЕФЫЎШмвКЗЂЩњШЁДњЗДгІЩњГЩДМBЃЌBБЛбѕЦјбѕЛЏЩњГЩвьЖЁШЉЃЌдђBЪЧ2-МзЛљ-1-БћДМЃЌAЪЧ2-МзЛљ-1-фхБћЭщЃЌвьЖЁШЉКЭCЗДгІЩњГЩDЃЌDЫЎНтЩњГЩввДМКЭEЃЌИљОнЬтИјаХЯЂжЊЃЌEКЭЧтЦјЗЂЩњМгГЩЗДгІЩњГЩFЃЌFМгШШЗжНтЩњГЩЫЎКЭGЃЌИљОнGЕФНсЙЙМђЪНжЊЃЌFЕФНсЙЙМђЪНЮЊЃКHOCH2 CЃЈCH3ЃЉ2CHOHCOOHЃЌEЕФНсЙЙМђЪНЮЊЃКOHCCЃЈCH3ЃЉ2CHOHCOOHЃЌDЕФНсЙЙМђЪНЮЊЃКOHCCЃЈCH3ЃЉ2CHOHCOOCH2CH3ЃЌCЕФНсЙЙМђЪНЮЊЃКOHCCOOCH2CH3ЃЌ
ЃЈ1ЃЉИљОнGЕФНсЙЙМђЪНжЊЃЌGЕФЗжзгЪНЮЊC6H10O3ЃЌDЕФНсЙЙМђЪНЮЊOHCCЃЈCH3ЃЉ2CHOHCOOCH2CH3ЃЌКЌгаЕФЙйФмЭХЪЧѕЅЛљЁЂШЉЛљЁЂєЧЛљЃЛ
ЃЈ2ЃЉЕкЂкВНТБДњЬўЗЂЩњЕФЫЎНтЗДгІЕФЛЏбЇЗНГЬЪНЮЊ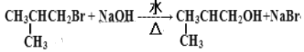 ЃЛ
ЃЛ
ЃЈ3ЃЉЕкЂлВНЗДгІЕФЛЏбЇЗНГЬЪНЮЊ![]() ЃЛ
ЃЛ
ЃЈ4ЃЉаДГіFЕФНсЙЙМђЪНЃКHOCH2 CЃЈCH3ЃЉ2CHOHCOOHЁЃ
ЃЈ5ЃЉЕкЂй~ЂоВНЗДгІжаЂйЪЧМгГЩЗДгІЃЌЂкЪЧШЁДњЗДгІЃЌЂлЪЧбѕЛЏЗДгІЃЌЂмМгГЩЗДгІЃЌЂнШЁДњЗДгІЃЌЂоМгГЩЗДгІЃЌЫљвдЪєгкМгГЩЗДгІЕФгаЂйЂмЂоЃЌШЁДњЗДгІЕФгаЂкЂнЃЛ
ЃЈ6ЃЉЭЌЪБТњзуЬѕМўЕФEЕФЭЌЗжвьЙЙЬхгаЃКCH3COOCH2CH2OOCCH3ЁЂCH3CH2OOCCOOCH2CH3ЁЂCH3OOCCH2CH2COOCH3 ЃЌЙВ3жжЁЃ


 ЪРМЭАйЭЈЦкФЉН№ОэЯЕСаД№АИ
ЪРМЭАйЭЈЦкФЉН№ОэЯЕСаД№АИ
| ФъМЖ | ИпжаПЮГЬ | ФъМЖ | ГѕжаПЮГЬ |
| ИпвЛ | ИпвЛУтЗбПЮГЬЭЦМіЃЁ | ГѕвЛ | ГѕвЛУтЗбПЮГЬЭЦМіЃЁ |
| ИпЖў | ИпЖўУтЗбПЮГЬЭЦМіЃЁ | ГѕЖў | ГѕЖўУтЗбПЮГЬЭЦМіЃЁ |
| ИпШ§ | ИпШ§УтЗбПЮГЬЭЦМіЃЁ | ГѕШ§ | ГѕШ§УтЗбПЮГЬЭЦМіЃЁ |
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПЃЈ1ЃЉгаЯТСаМИзщЮяжЪЃЌЧыНЋађКХЬюШыЯТСаПеИёФкЃК
AЁЂCH2=CH-COOHКЭгЭЫсЃЈC17H33COOHЃЉ BЁЂ12C60КЭЪЏФЋ
CЁЂ![]() КЭ
КЭ![]() DЁЂ35ClКЭ37Cl EЁЂввДМКЭввЖўДМ
DЁЂ35ClКЭ37Cl EЁЂввДМКЭввЖўДМ
ЂйЛЅЮЊЭЌЮЛЫиЕФЪЧ ЃЛ
ЂкЛЅЮЊЭЌЯЕЮяЕФЪЧ ЃЛ
ЂлЛЅЮЊЭЌЫивьаЮЬхЕФЪЧ ЃЛ
ЂмЛЅЮЊЭЌЗжвьЙЙЬхЕФЪЧ ЃЛ
ЂнМШВЛЪЧЭЌЯЕЮяЃЌгжВЛЪЧЭЌЗжвьЙЙЬхЃЌвВВЛЪЧЭЌЫивьаЮЬхЃЌЕЋПЩПДГЩЪЧЭЌвЛРрЮяжЪЕФЪЧ ЁЃ
ЃЈ2ЃЉЧыаДГіЯТСаЗДгІЕФЛЏбЇЗНГЬЪНЃК
ЂйгЩБћЯЉжЦШЁОлБћЯЉЃК ЃЛЃЛ
ЂкБћАБЫсЫѕОлаЮГЩЖрыФЃК ЃЛ
ЂлЕэЗлЫЎНтЃК ЃЛ
ЂмБћШЉгыаТжЦЕФЧтбѕЛЏЭаќзЧвКЗДгІЃК ЃЛ
ЃЈ3ЃЉгУвЛжжЪдМСНЋЯТСаИїзщЮяжЪМјБ№ПЊЃЌаДГіЦфУћГЦЁЃ
Ђй![]() КЭ
КЭ![]() ЃК ЃЛ
ЃК ЃЛ
Ђк ![]() ЃЌ
ЃЌ![]() КЭC6H12(МКЯЉ)ЃК ЃЛ
КЭC6H12(МКЯЉ)ЃК ЃЛ
Ђл ![]() ЃЌCCl4КЭввДМ ЁЃ
ЃЌCCl4КЭввДМ ЁЃ
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПБНМзЫсФЦЃЈ![]() ЃЌЫѕаДЮЊNaAЃЉПЩгУзївћСЯЕФЗРИЏМСЁЃбаОПБэУїБНМзЫсЃЈHAЃЉЕФвжОњФмСІЯджјИпгкAЈCЁЃвбжЊ25 ЁцЪБЃЌHAЕФKa=6.25ЁС10ЈC5ЃЌH2CO3ЕФKa1=4.17ЁС10ЈC7ЃЌKa2=4.90ЁС10ЈC11ЁЃдкЩњВњЬМЫсвћСЯЕФЙ§ГЬжаЃЌГ§СЫЬэМгNaAЭтЃЌЛЙашМгбЙГфШыCO2ЦјЬхЁЃЯТСаЫЕЗЈе§ШЗЕФЪЧЃЈЮТЖШЮЊ25 ЁцЃЌВЛПМТЧвћСЯжаЦфЫћГЩЗжЃЉЃЈ ЃЉ
ЃЌЫѕаДЮЊNaAЃЉПЩгУзївћСЯЕФЗРИЏМСЁЃбаОПБэУїБНМзЫсЃЈHAЃЉЕФвжОњФмСІЯджјИпгкAЈCЁЃвбжЊ25 ЁцЪБЃЌHAЕФKa=6.25ЁС10ЈC5ЃЌH2CO3ЕФKa1=4.17ЁС10ЈC7ЃЌKa2=4.90ЁС10ЈC11ЁЃдкЩњВњЬМЫсвћСЯЕФЙ§ГЬжаЃЌГ§СЫЬэМгNaAЭтЃЌЛЙашМгбЙГфШыCO2ЦјЬхЁЃЯТСаЫЕЗЈе§ШЗЕФЪЧЃЈЮТЖШЮЊ25 ЁцЃЌВЛПМТЧвћСЯжаЦфЫћГЩЗжЃЉЃЈ ЃЉ
AЃЎЯрБШгкЮДГфCO2ЕФвћСЯЃЌЬМЫсвћСЯЕФвжОњФмСІНЯЕЭ
BЃЎЬсИпCO2ГфЦјбЙСІЃЌвћСЯжаc(AЈC)ВЛБф
CЃЎЕБpHЮЊ5.0ЪБЃЌвћСЯжа![]() =0.16
=0.16
DЃЎЬМЫсвћСЯжаИїжжСЃзгЕФХЈЖШЙиЯЕЮЊЃКc(H+)=c(![]() )+c(
)+c(![]() )+c(OHЈC)ЈCc(HA)
)+c(OHЈC)ЈCc(HA)
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПЯТСагаЙиЮяжЪЕФаджЪгыгІгУЯрЖдгІЕФЪЧ
AЃЎSO2ОпгабѕЛЏадЃЌПЩгУгкЦЏАзжННЌ
BЃЎЧтЗњЫсОпгаЫсадЃЌПЩгУгкЕёПЬВЃСЇ
CЃЎЖўбѕЛЏТШОпгаЛЙдадЃЌПЩгУгкздРДЫЎЕФЩБОњЯћЖО
DЃЎNH3ОпгаЛЙдадЃЌПЩгУNH3гызЦШШCuOзїгУжЦШЁЩйСПN2
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПЯђФГУмБеШнЦїжаГфШы1 mol COКЭ2 mol H2O(g)ЃЌЗЂЩњЗДгІЃКCOЃЋH2O(g)![]() CO2ЃЋH2ЁЃЕБЗДгІДяЕНЦНКтЪБЃЌCOЕФЬхЛ§ЗжЪ§ЮЊxЁЃШєЮЌГжШнЦїЕФЬхЛ§КЭЮТЖШВЛБфЃЌЦ№ЪМЮяжЪАДЯТСаЫФжжХфБШГфШыИУШнЦїжаЃЌДяЕНЦНКтЪБCOЕФЬхЛ§ЗжЪ§ДѓгкxЕФЪЧ(ЁЁЁЁ)
CO2ЃЋH2ЁЃЕБЗДгІДяЕНЦНКтЪБЃЌCOЕФЬхЛ§ЗжЪ§ЮЊxЁЃШєЮЌГжШнЦїЕФЬхЛ§КЭЮТЖШВЛБфЃЌЦ№ЪМЮяжЪАДЯТСаЫФжжХфБШГфШыИУШнЦїжаЃЌДяЕНЦНКтЪБCOЕФЬхЛ§ЗжЪ§ДѓгкxЕФЪЧ(ЁЁЁЁ)
A. 0.5 mol COЃЋ2 mol H2O(g)ЃЋ1 mol CO2ЃЋ1 mol H2
B. 1 mol COЃЋ1 mol H2O(g)ЃЋ1 mol CO2ЃЋ1 mol H2
C. 0.5 mol COЃЋ1.5 mol H2O(g)ЃЋ0.4 mol CO2ЃЋ0.4 mol H2
D. 0.5 mol COЃЋ1.5 mol H2O(g)ЃЋ0.5 mol CO2ЃЋ0.5 mol H2
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПвбжЊAЁЂBЁЂCЁЂDЪЧдзгађЪ§вРДЮдіДѓЕФЫФжжЖЬжмЦкжїзхдЊЫиЃЌAЕФжмЦкЪ§ЕШгкЦфжїзхађЪ§ЃЌBдзгЕФМлЕчзгХХВМЮЊnsnnpnЃЌDЪЧЕиПЧжаКЌСПзюЖрЕФдЊЫиЁЃEЪЧЕкЫФжмЦкpЧјЕФдЊЫиЧвзюЭтВужЛга2ЖдГЩЖдЕчзгЃЌFЪЧ29КХдЊЫиЁЃ
ЃЈ1ЃЉBЁЂCЁЂDШ§дЊЫиЕквЛЕчРыФмгЩДѓЕНаЁЕФЫГађЮЊ ЃЈгУдЊЫиЗћКХБэЪОЃЉ
ЃЈ2ЃЉBD32-жааФдзгдгЛЏЙьЕРЕФРраЭЮЊ________дгЛЏЃЛCA4+ЕФПеМфЙЙаЭЮЊ______________ЁЃ
ЃЈ3ЃЉЛљЬЌEдзгЕФМлЕчзгХХВМЭМ______________________________ЁЃ
ЃЈ4ЃЉ1mol BCЃжаКЌгаІаМќЕФЪ§ФПЮЊ______________ЁЃ
ЃЈ5ЃЉБШНЯDЁЂEдЊЫизюМђЕЅЧтЛЏЮяЕФЗаЕуИпЕЭЃК ЃЈгУЛЏбЇЪНБэЪОЃЉЁЃ
ЃЈ6ЃЉCЁЂFСНдЊЫиаЮГЩЕФФГЛЏКЯЮяЕФОЇАћНсЙЙШчЭМЫљЪОЃЌЖЅЕуЮЊCдзгЁЃдђИУЛЏКЯЮяЕФЛЏбЇЪНЪЧ ЃЌCдзгЕФХфЮЛЪ§ЪЧ ЁЃШєЯрСкCдзгКЭFдзгМфЕФОрРыЮЊa cmЃЌАЂЗќйЄЕТТоГЃЪ§ЮЊNAЃЌдђИУОЇЬхЕФУмЖШЮЊ________________gЃЏcm3ЃЈгУКЌaЁЂNAЕФЗћКХБэЪОЃЉЁЃ

ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПAЁЂBЁЂCЁЂDЮЊдзгађЪ§вРДЮдіДѓЕФЫФжждЊЫиЃЌA2ЃКЭB+ОпгаЯрЭЌЕФЕчзгЙЙаЭЃЛCЁЂ DЮЊЭЌжмЦкдЊЫїЃЌCКЫЭтЕчзгзмЪ§ЪЧзюЭтВуЕчзгЪ§ЕФ3БЖЃЛDдЊЫизюЭтВугавЛИіЮДГЩЖдЕчзгЁЃЛиД№ЯТСаЮЪЬтЃК
ЃЈ1ЃЉЫФжждЊЫижаЕчИКадзюаЁЕФЪЧ________ЃЈЬюдЊЫиЗћКХЃЉЃЌЦфжаCдзгЕФЭтЮЇЕчзгХХВМЭМЮЊ________ЁЃ
ЃЈ2ЃЉAКЭBЕФЧтЛЏЮяЫљЪєЕФОЇЬхРраЭЗжБ№ЮЊ_________КЭ_________ЁЃ
ЃЈ3ЃЉBЁЂCОљПЩвдгыDаЮГЩЛЏКЯЮяЃЌЦфжаШлЕуНЯИпЕФЪЧ____ЃЈгУЛЏбЇЪНБэЪОЃЉ
ЃЈ4ЃЉAКЭBПЩаЮГЩ1:1аЭЕФЛЏКЯЮяEЃЌEЕФЕчзгЪНЮЊ_____
ЃЈ5ЃЉЛЏКЯЮяD2AЕФСЂЬхЙЙаЭЮЊ_________ЃЌжааФдзгЕФЙТЕчзгЖдЪ§ЮЊ_________ЃЌЕЅжЪDгыЪЊШѓЕФNa2CO3ЗДгІПЩжЦБИD2AЃЌЦфЛЏбЇЗНГЬЪНЮЊ_______________ЁЃ
ЃЈ6ЃЉAКЭBФмЙЛаЮГЩЛЏКЯЮяFЃЌЦфОЇАћНсЙЙШчЭМЫљЪОЃЌОЇАћБпГЄЮЊ0.566nmЃЌ F ЕФЛЏбЇЪНЮЊ______ЃЛОЇАћжаA дзгЕФХфЮЛЪ§ЮЊ______ЃЛОЇЬхFЕФУмЖШ=______gЃЎcmЃ3ЃЈжЛСаЪНЃЌВЛМЦЫуЃЉ

ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПАыЫЎУКЦјЪЧЙЄвЕКЯГЩАБЕФдСЯЦјЃЌЦфжївЊГЩЗжЪЧH2ЁЂCOЁЂCO2ЁЂN2КЭH2OЃЈgЃЉЁЃАыЫЎУКЦјОЙ§ЯТСаВНжшзЊЛЏЮЊКЯГЩАБЕФдСЯЁЃ

ЭъГЩЯТСаЬюПеЃК
ЃЈ1ЃЉАыЫЎУКЦјКЌгаЩйСПСђЛЏЧтЁЃНЋАыЫЎУКЦјбљЦЗЭЈШы____ШмвКжаЃЈЬюаДЪдМСУћГЦЃЉЃЌГіЯж_______ЃЌПЩвджЄУїгаСђЛЏЧтДцдкЁЃ
ЃЈ2ЃЉАыЫЎУКЦјдкЭДпЛЏЯТЪЕЯжCOБфЛЛЃКCO+H2O![]() CO2+H2
CO2+H2
ШєАыЫЎУКЦјжаVЃЈH2ЃЉ:VЃЈCOЃЉ:VЃЈN2ЃЉ=38ЃК28ЃК22ЃЌОCOБфЛЛКѓЕФЦјЬхжаЃКVЃЈH2ЃЉ:VЃЈN2ЃЉ=____________ЁЃ
ЃЈ3ЃЉМювКЮќЪеЗЈЪЧЭбГ§ЖўбѕЛЏЬМЕФЗНЗЈжЎвЛЁЃвбжЊЃК
Na2CO3 | K2CO3 | |
20ЁцМювКзюИпХЈЖШЃЈmol/LЃЉ | 2.0 | 8.0 |
МюЕФМлИёЃЈдЊ/kgЃЉ | 1.25 | 9.80 |
ШєбЁдёNa2CO3МювКзїЮќЪевКЃЌЦфгХЕуЪЧ__________ЃЛШБЕуЪЧ____________ЁЃШчЙћбЁдёK2CO3МювКзїЮќЪевКЃЌгУЪВУДЗНЗЈПЩвдНЕЕЭГЩБОЃП
___________________________________________
аДГіетжжЗНЗЈЩцМАЕФЛЏбЇЗДгІЗНГЬЪНЁЃ_______________________
ЃЈ4ЃЉвдЯТЪЧВтЖЈАыЫЎУКЦјжаH2вдМАCOЕФЬхЛ§ЗжЪ§ЕФЪЕбщЗНАИЁЃ
ШЁвЛЖЈЬхЛ§ЃЈБъзМзДПіЃЉЕФАыЫЎУКЦјЃЌОЙ§ЯТСаЪЕбщВНжшВтЖЈЦфжаH2вдМАCOЕФЬхЛ§ЗжЪ§ЁЃ

ЂйбЁгУКЯЪЪЕФЮоЛњЪдМСЗжБ№ЬюШыЂёЁЂЂёЁЂЂєЁЂЂѕЗНПђжаЁЃ
ЂкИУЪЕбщЗНАИжаЃЌВНжшЂёЁЂЂђЕФФПЕФЪЧЃК ЁЃ
ЂлИУЪЕбщЗНАИжаЃЌВНжш________ЃЈбЁЬюЁАЂєЁБЛђЁАЂѕЁБЃЉПЩвдШЗЖЈАыЫЎУКЦјжаH2ЕФЬхЛ§ЗжЪ§ЁЃ
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПЃЈ1ЃЉШчЭМЫљЪОзАжУЃЌзмЗДгІЕФРызгЗНГЬЪНЮЊ_________________________ЁЃ

ЃЈ2ЃЉжЪСПЯрЕШЕФСНЕчМЋЗДгІКѓжЪСПЯрВю12 gЃЌдђЕМЯпжаЭЈЙ§СЫ____________mol ЕчзгЁЃ
ЃЈ3ЃЉЦфЫћЬѕМўВЛБфЃЌШєНЋCuCl2ШмвКЛЛЮЊNH4ClШмвКЃЌЪЏФЋЕчМЋЗДгІЪН_____________________ЃЌетЪЧгЩгкNH4ClШмвКЯд__________ (ЬюЁАЫсадЁБЁАМюадЁБЛђЁАжаадЁБ)ЃЌгУРызгЗНГЬЪНБэЪОШмвКЯдДЫадЕФдвђ____________________ЁЃ
ВщПДД№АИКЭНтЮі>>
ЙњМЪбЇаЃгХбЁ - СЗЯАВсСаБэ - ЪдЬтСаБэ
КўББЪЁЛЅСЊЭјЮЅЗЈКЭВЛСМаХЯЂОйБЈЦНЬЈ | ЭјЩЯгаКІаХЯЂОйБЈзЈЧј | ЕчаХеЉЦОйБЈзЈЧј | ЩцРњЪЗащЮожївхгаКІаХЯЂОйБЈзЈЧј | ЩцЦѓЧжШЈОйБЈзЈЧј
ЮЅЗЈКЭВЛСМаХЯЂОйБЈЕчЛАЃК027-86699610 ОйБЈгЪЯфЃК58377363@163.com