
����Ŀ���ߴ�������[Sr(NO3)2]�����������źŵơ���ѧ�����ȡ���ҵ�������Ⱥ�����ơ����ᱵ�����ʣ��ᴿ�������£�
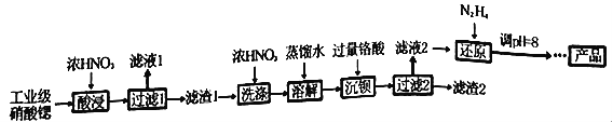
��֪��������Һ1������Ҫ������Ca(NO3)2��������1���ijɷ�ΪBa(NO3)2��Sr(NO3)2��������2������Ҫ�ɷ�Ϊ BaCrO4(���ʲ������ᷴӦ)���ڸ���(H2CrO4)Ϊ���ᡣ
��1������������ܲ��ø��µ�ԭ����_________________________________��
��2�������ˮϴ����ŨHNO3ϴ�ӵ��ŵ���_________________________________��
��3������Һ2���й�����H2CrO4��N2H4��ԭΪCr3+��ͬʱ�ų�����Ⱦ�����壬д����Ӧ�����ӷ���ʽ_______________________________________________________��
��4������Һ�д������³����ܽ�ƽ�⣺Cr(OH)3(s)![]() Cr3+(aq)+3OH��(aq)�������£�Cr(OH)3���ܶȻ�Ksp=1.0��10��32����c(Cr3+)����1.0��10��5mol/L����ΪCr3+�Ѿ���ȫ�������ֽ���ԭ����Һ��pHֵ����4����ʱCr3+�Ƿ������ȫ?______________________(��ʽ����)��
Cr3+(aq)+3OH��(aq)�������£�Cr(OH)3���ܶȻ�Ksp=1.0��10��32����c(Cr3+)����1.0��10��5mol/L����ΪCr3+�Ѿ���ȫ�������ֽ���ԭ����Һ��pHֵ����4����ʱCr3+�Ƿ������ȫ?______________________(��ʽ����)��
��5����֪Cr(OH)3����Al(OH)3����ԭ����Һ��pH���ܴ���8��ԭ���ǣ�___________��(������ӷ���ʽ˵������)��
��6��Ϊ�˲ⶨ������2���� BaCrO4�ĺ�������������ʵ�飺
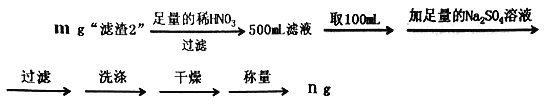
���ж�Ba2+��ȫ�����ķ�����____________________________________________��
��������2����BaCO4����������Ϊ______________________(�ô���ʽ��ʾ)��
���𰸡�����ŨHNO3�ӷ��ͷֽ� ����Sr(NO3)2�ܽ�������ʧ 4H2CrO4+3N2H4+12H+=4Cr3++3N2+16H2O c(OH-)=10-10mol/Lʱ��c(Cr3+)=1.0��10-32/(1.0��10-10)3=1.0��10-2mol/L>1.0��10-5mol/L����ʱCr3+���ܳ�����ȫ pH����8ʱCr(OH)3���ܽ���Cr(OH)3+ OH-=CrO2-+2H2O ���ã����ϲ���Һ�м����μ�Na2SO4��Һ���ϲ���Һ���ް�ɫ�������� ![]() ��100%
��100%
��������
��1��HNO3�ӷ��ͷֽ���
��2����������ŨHNO3�б���ˮ���ܽ��С��
��3��H2CrO4��N2H4��ԭΪCr3+��ͬʱ�ų�����Ⱦ������ΪN2������������ԭ��Ӧ����ʽ����ƽ��
��4������Ksp[Cr(OH)3]= c(Cr3+)![]() c3(OH-)=1.0��10��32��c(OH-)=10-10mol/Lʱ��c(Cr3+)=1.0��10-32/(1.0��10-10)3=1.0��10-2mol/L>1.0��10-5mol/L����ʱCr3+���ܳ�����ȫ��
c3(OH-)=1.0��10��32��c(OH-)=10-10mol/Lʱ��c(Cr3+)=1.0��10-32/(1.0��10-10)3=1.0��10-2mol/L>1.0��10-5mol/L����ʱCr3+���ܳ�����ȫ��
��5����֪Cr(OH)3����Al(OH)3��Cr(OH)3�ڼ�����Һ�лᷢ��Cr(OH)3+ OH-=CrO2-+2H2O���ܽ⣻
��6���ٸ��������ƺ����ᱵ��Ӧ�������ᱵ������������з�����
�ڸ������ᱵ��������������2�����������м�����
��1��HNO3�ӷ��ͷֽ�������������ܲ��ø��µ�ԭ���DZ���HNO3�ӷ��ͷֽ���
�ʴ�Ϊ������HNO3�ӷ��ͷֽ���
��2������ͬ����ЧӦ�������ˮϴ����������ŨHNO3�б���ˮ���ܽ��С����ŨHNO3ϴ�ӵ��ŵ��Ǽ���������(������Ʒ��)�ܽ���ʧ��
�ʴ�Ϊ������Sr(NO3)2�ܽ�������ʧ��
��3������Һ2���й�����H2CrO4��N2H4��ԭΪCr3+��Cr�Ļ��ϼ۱仯��+6��+3������N�Ļ��ϼ����ߣ�ͬʱ����һ����ɫ��ζ�����壬Ϊ������N�Ļ��ϼ۱仯��-2��0����ת�Ƶ�����С������Ϊ��12�����ݵ�ʧ�����غ��ԭ���غ㣬���ӷ���ʽΪ��4H2CrO4+3N2H4+12H+=4Cr3++3N2+16H2O��
�ʴ�Ϊ��4H2CrO4+3N2H4+12H+=4Cr3++3N2+16H2O��
��4������Ksp[Cr(OH)3]= c(Cr3+)![]() c3(OH-)=1.0��10��32��c(OH-)=10-10mol/Lʱ��c(Cr3+)=1.0��10-32/(1.0��10-10)3=1.0��10-2mol/L>1.0��10-5mol/L����ʱCr3+���ܳ�����ȫ��
c3(OH-)=1.0��10��32��c(OH-)=10-10mol/Lʱ��c(Cr3+)=1.0��10-32/(1.0��10-10)3=1.0��10-2mol/L>1.0��10-5mol/L����ʱCr3+���ܳ�����ȫ��
�ʴ�Ϊ��c(OH-)=10-10mol/Lʱ��c(Cr3+)=1.0��10-32/(1.0��10-10)3=1.0��10-2mol/L>1.0��10-5mol/L����ʱCr3+���ܳ�����ȫ��
��5����֪Cr(OH)3����Al(OH)3��Cr(OH)3�ڼ�����Һ�лᷢ��Cr(OH)3+ OH-=CrO2-+2H2O���ܽ������Ի�ԭ����Һ��pH���ܴ���8��
�ʴ�Ϊ��pH����8ʱCr(OH)3���ܽ���Cr(OH)3+ OH-=CrO2-+2H2O��
��6�������ᱵҺ��Na2SO4��Һ��Ӧ�������ᱵ���Ȼ��ƣ������ж�Ba2+��ȫ�����ķ����ǣ����ã����ϲ���Һ�м����μ�Na2SO4��Һ���ϲ���Һ���ް�ɫ�������ɣ�
����������2����BaCrO4������Ϊx��
BaCrO4~~~BaSO4
253 233
X ng��5![]() =
=![]() �����x=
�����x=![]() g
g
����������2����BaCO4����������Ϊ:![]() ��100%=
��100%=![]() ��100%��
��100%��
�ʴ�Ϊ�����ã����ϲ���Һ�м����μ�Na2SO4��Һ���ϲ���Һ���ް�ɫ�������ɣ�![]() ��100%��
��100%��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���л���Ľṹ���á�����ʽ����ʾ����CH3��CH=CH��CH3���ɼ�дΪ![]() ���л���X�ļ���ʽΪ
���л���X�ļ���ʽΪ![]() ������˵������ȷ����( )
������˵������ȷ����( )
A. X�Ļ�ѧʽΪC8H8
B. �л���Y��X��ͬ���칹�壬�����ڷ���������Y�Ľṹ��ʽ����Ϊ![]()
C. X��ʹ�������������Һ��ɫ
D. X��������H2��һ�������·�Ӧ�����ɻ�״�ı�����Z��Z��һ�ȴ�����4��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�������й�ʵ��װ�á�����������ʵ����Ӧʵ��Ŀ�ĵ���
A | B | C | D | |
װ�� |
|
|
|
|
Ŀ�� | ��KOH��Һ��ȥ�屽�е����� | ֤��Ũ��������ˮ�ԡ�ǿ������ | �ȳ��ְ�ɫ�����������ש��ɫ������֤��Ksp(AgCl)< Ksp(Ag2CrO4) | ����ͨ���۲�ˮ�ܷ�ȫ���������ж�װ�������� |
A. A B. B C. C D. D
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ѧ��ѧѧϰ����һЩ�����������ͼװ�÷ֱ���ȡ��������ռ����ձ��е�NaOH��Һ��������β��������Ҫ��ش��������⡣

��1����������ø�װ����ȡ������ռ������յ�����Է�Ӧ���Ӧ�������������ʱ�����ϵ�Ҫ��
�ٶԷ�Ӧ���Ҫ��____________��
�ڶԷ�Ӧ������Ҫ��___________��
�۶������������ʵ�Ҫ��___________��
��2��ʵ�����ø�����غ�Ũ��������ͼװ������ȡ��������ش�
��B��ʢ�ŵ�ҩƷ������_____��
��C�е�������________����C�г���______������ʱ��˵����Ӧ��û���������ɡ�
��NaOH��Һ��������_______����Ӧ�Ļ�ѧ����ʽΪ___��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��������,��10 mL 0.1 mol��L-1 Na2CO3��Һ����μ���0.1 mol��L-1 HCl��Һ,��Һ��pH����,��ʱ��Һ�к�̼�������ʵ��������仯��ͼ��ʾ(CO2���ݳ�δ����,�����������ݳ��������Һ����仯),����˵����ȷ����

A. ��0.1 mol/L Na2CO3��Һ��:c(Na+)+c(H+)=c(CO32��)+c(HCO3��)+c(OH��)
B. ����Һ��pHΪ7ʱ,��Һ�������Ϊ20 mL
C. ��B����ʾ����Һ��,Ũ��������������Na+
D. �� A����ʾ����Һ��:c(CO32��)=c(HCO3��)>c(H+)>c(OH��)
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����������ϵ����ȷ���ǣ� ��
ѡ�� | ԭ�� | ��� |
A | ����ֲ������ | ����ЧӦ |
B | SO2��NO2����Ĵ����ŷ� | ���� |
C | �������������ˮ�Ĵ����ŷ� | ˮ�����ೱ |
D | ����β���Ĵ����ŷ� | �⻯ѧ���� |
A. AB. BC. CD. D
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���������������������Ԫ�ء�
��1�������к��зḻ���Ե�������ʽ���ڵĵ�Ԫ�ء���ʵ�����У��Ӻ�������ȡ����������£�

�������������漰���в��������д������________________�����ţ���
A.���������ճɻ�
B.���˺�I-��Һ
C���¿ڷų�����ı���Һ
D������Ⲣ���ձ�
��д������ܷ�����Ӧ�����ӷ���ʽ��_______________________________________
��Ҫ֤�������������Һ�к��еⵥ�ʣ��ɼ���___________________�����Լ����ƣ����۲쵽________________________��������˵����Һ�д��ڵ⡣
��2��̽������ϡ����ķ�Ӧ����5.6gFe�ۺͺ���0.3 mol HNO3��ϡ�������ʵ�飬����������ǡ����ȫ��Ӧ����HNO3ֻ����ԭ��NO���ش��������⣺
��Fe����ϡ���ᷴӦ�����������Ļ�ѧ����ʽΪ___________________________________
�ڷ�Ӧ���������Һ��Fe3����Fe2�������ʵ���֮��n��Fe3������n��Fe2������_________________��
�۱�״���£�����NO��������Ϊ_________________L��������λС������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����(CN)2�Ľṹ��ʽΪN��C��C��N���仯ѧ������±��(X2)�����ƣ���ѧ�ϳ�֮Ϊ��±�أ��������Խ���Br2��I2֮�䡣�����йط�Ӧ�Ļ�ѧ����ʽ����ȷ����(����)
A. (CN)2��H2=2HCN
B. MnO2��4HCN=Mn(CN)2��(CN)2����2H2O
C. ��KCN��Һ�м����ˮ��I2��2KCN=2KI��(CN)2
D. ��NaBr��KCN���Һ��ͨ������Cl2��Cl2��2KCN=2KCl��(CN)2
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��Ϊ�˱���NO��NO2��N2O4�Դ�������Ⱦ��������NaOH��Һ�������մ���(��Ӧ����ʽ��2NO2��2NaOH===NaNO3��NaNO2��H2O��NO2��NO��2NaOH===2NaNO2��H2O)��������a mol NO��b mol NO2��c mol N2O4��ɵĻ������ǡ�ñ�V L NaOH��Һ����(������ʣ��)�����NaOH��Һ�����ʵ���Ũ��Ϊ(����)
A. ![]() mol��L��1 B.
mol��L��1 B. ![]() mol��L��1
mol��L��1
C. ![]() mol��L��1 D.
mol��L��1 D. ![]() mol��L��1
mol��L��1
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com