
(15��)ij��ѧ��ȤС���������¸�װ�����ӳ�һ����װ�ã�̽�������백��֮��ķ�Ӧ������DΪ��������������봿�����ﰱ����Ӧ��װ�ã�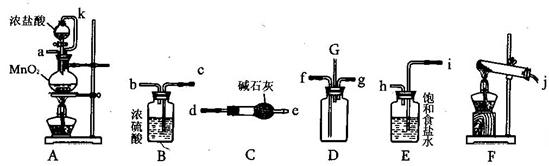
��ش��������⣺
(1)���Ӻ�װ�ú�����е�һ��ʵ�������__________________
(2)װ��E��������____________����k��������________________________
(3)��װ��D��G���ݳ���β���п��ܺ��л���ɫ���ж����壬����������__________________
(4)װ��F���Թ��ڷ�����Ӧ�Ļ�ѧ����ʽ____________________________________
(5)����Dװ�õ�����������߽ϳ����ұ߽϶̣�Ŀ����______________________________
(6)����װ�ô������ҵ�����˳����(j)��( ) ( )��(f) (g)��( ) ( )��( ) ( )��(a)��
��1�����װ�õ������ԣ�1�֣�
��2����ȥ�����е��Ȼ��⣨2�֣�ʹ��Һ©���е�����˳�����£�2�֣�
��3����G���ӵ���ֱ��ͨ��ʢ���ռ���ձ��У�2�֣�
��4��2NH4Cl��Ca(OH)2 CaCl2��2H2O��2NH3����3�֣�
CaCl2��2H2O��2NH3����3�֣�
��5��ʹ�ܶȴ���������ܶ�С�İ����Ͽ�ؾ��Ȼ�ϣ�2�֣�
��6����j���ӣ�d����e���ӣ�f����g���ӣ�b����c���ӣ�h����i���ӣ�a����3�֣�ȫ�Բŵ÷֣�
����������������⿼�鴿��������������Ͱ������Ʊ�������֮��ķ�Ӧ��AΪ�����ķ���װ�ã�Eװ�����ڳ�ȥ�����е��Ȼ��⣬Bװ��Ϊ�����ĸ���װ�ã�Fװ��Ϊ�����ķ���װ�ã�Cװ��Ϊ�����ĸ���װ�ã�Dװ��Ϊ��������ķ�Ӧװ�á�(1)���Ӻ�װ�ú�����е�һ��ʵ������Ǽ��װ�õ������ԣ�(2)װ��E�������dz�ȥ�����е��Ȼ��⣬��k��������ʹ��Һ©���е�����˳�����£�(3)��װ��D��G���ݳ���β���п��ܺ��л���ɫ���ж����壬������������G���ӵ���ֱ��ͨ��ʢ���ռ���ձ��У�(4)װ��F���Թ��ڷ�����Ӧ�Ļ�ѧ����ʽ2NH4Cl��Ca(OH)2 CaCl2��2H2O��2NH3����(5) ���ڰ������ܶ�С���������ܶȴ��ܶ�С�����Ĵӳ��ܽ���������ɢ���ܶȴ�������Ӷ̹ܽ���������ɢ������Dװ�õ�����������߽ϳ����ұ߽϶̣�Ŀ����ʹ�ܶȴ���������ܶ�С�İ����Ͽ�ؾ��Ȼ�ϣ�(6)����װ�ô������ҵ�����˳���ǣ�j���ӣ�d����e���ӣ�f����g���ӣ�b����c���ӣ�h����i���ӣ�a����
CaCl2��2H2O��2NH3����(5) ���ڰ������ܶ�С���������ܶȴ��ܶ�С�����Ĵӳ��ܽ���������ɢ���ܶȴ�������Ӷ̹ܽ���������ɢ������Dװ�õ�����������߽ϳ����ұ߽϶̣�Ŀ����ʹ�ܶȴ���������ܶ�С�İ����Ͽ�ؾ��Ȼ�ϣ�(6)����װ�ô������ҵ�����˳���ǣ�j���ӣ�d����e���ӣ�f����g���ӣ�b����c���ӣ�h����i���ӣ�a����
���㣺����������Ʊ�������ʵ�顣


 ��ʦ����ָ���ο�ʱϵ�д�
��ʦ����ָ���ο�ʱϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
���仯��������Ȼ���ձ���ڡ�����ת����
��ɽ�緢�������ʽ���ʱ�������ж�����H2S��SO2�Ƚ����������һЩ��Ȼת����������������
��1��H2S��SO2������ײʱ����Ⱦ�ή�͡���Ӧ����ʽΪ_________________________��
��2��H2S�ڳ����£��ᱻ��������������Ӧ����ʽΪ_________________________��
��3��SO2��Ʈ�����£��ᱻ��������ΪSO3����ˮ�������ᣬ������ʯ����ת��Ϊ�ȶ���ʯ�����(CaSO4��2H2O)��SO2�������Ļ�ѧ����ʽΪ_____________________________��
�ڻ�����ʩ�ϣ��������Ǵ�����ʦ����Ȼ������ʾ��
����Ϊ�����ŷŶ�������ȣ��ᳬ����Ȼ���������������������ػ�����Ⱦ����ʯ��-ʯ�෨���͡��ռ���ǹ�ҵ�ϳ��õĹ�ҵ��������
��4����ʯ��-ʯ�෨�����ն������������Ϊ��
��SO2������ʯ����ܻ�ѧ����ʽΪ________________________________________��
��5�����ռ��������������Ũ��Һ���պ�������������������ŵ����������Ƽ���ǿ�����տ졢Ч�ʸߡ���ѧ����ʽΪ______________________________________�����ռ�ļ۸�Ϲ�ʯ��0��36Ԫ/kg���ռ�2��90Ԫ/kg����
��6��������ʯ��-ʯ�෨�� �͡��ռ����������������������������ٸ�Ч�ͳɱ�����Ⱦ�ĺ�Ч���������Եõ�ʯ���Ʒ��������������ͼ�Ģ١��ܴ���д���ʵĻ�ѧʽ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�������
��ͭƬ�м���115mLijŨ�ȵ����ᣬ�ڼ��������·�Ӧ����ͭƬȫ���ܽ������Һϡ�͵�500mL���ټ�������п�ۣ�ʹ֮��ַ�Ӧ���ռ���2��24L����״�������塣���˲������壬��������������������7��5g��
��1����μӷ�Ӧ��п�۵����ʵ���
��2��ԭ��������ʵ���Ũ�ȡ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
��15�֣����������μ����ֽ��Ҳ���ϸ��ӡ���Mg(NO3)2Ϊ�о������ĸ�ѧϰС����ͨ��ʵ��̽�����ȷֽ�IJ���ֱ��������4�ֲ��룺
���飺Mg(NO2)2��NO2��O2���� ���飺MgO��NO2��O2��
���飺MgO��NO2��N2�������� ���飺Mg3N2��O2
��1��ʵ��ǰ��С���Ա�������϶� ��IJ���һ����������������_____________��
�������ϵ�֪��2NO2+2NaOH=NaNO3+NaNO2+H2O
�������С����룬�������ͼ��ʾ��ʵ��װ�ã�ͼ�м��ȡ��г������Ⱦ�ʡ�ԣ�����̽����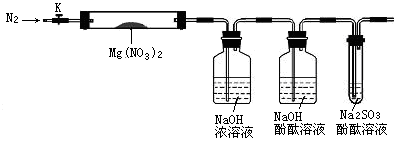
A B C D
��2��ʵ�����
�ټ��������װ�������Եķ��� ��
�ڳ�ȡӲ���Թ�A������Ϊ18.0g���Թ�A��Mg(NO3)2���干21.8 g����A�У�����ǰͨ��N2������װ���ڵĿ�������Ŀ���� ���ر�K���þƾ��Ƽ���ʱ����ȷ�������� Ȼ��̶��ڹ��й��岿λ�¼��ȡ�
�۹۲쵽A���к���ɫ�������ɣ�C�������ݡ�
�ܴ���Ʒ��ȫ�ֽ⣬Aװ����ȴ�����¡����������Ӳ���Թ�A��ʣ������������Ϊ19.0g��
��ȡ����ʣ��������Թ��У���������ˮ��δ����������
��3��ʵ������������
��֤��һ����O2���ɵ������� �������صĻ�ѧԭ��Ϊ���û�ѧ����ʽ��ʾ�� ��
����ʵ�������ʣ�����������������ɳ���ȷ�ϲ���_______���������ȷ�ġ�
��һλͬѧ��Ϊ����װ�ò���ȷ�Ϸֽ��������O2��������������Ϊ������ ���������Ľ�װ�ã�Ӧ�� ��
��4�������Ϸ�����Mg(NO3)2�ֽ�Ļ�ѧ����ʽΪ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
��10�֣�Ư�ۿ��Ժ�Ũ���ᷴӦ����������ij������ȤС����ͼ�ⶨ�������������֤�������������û��Ư���ԣ�������ͼ��ʾװ�ý���ʵ�飬
��ش��й����⣺
��1����װ�õ���ȷ����˳���ǣ�a��( )��( )��( )��( )��( )��( )��( )��
( )��( )��
��2��U�ܿ�������ʢװ__________��ϴ��ƿ����
��3������ȡ����ǰ��������е�һ�����������__________________
��4������ʵ��Ŀ�Ľ���֮������ɫ�����Ƿ���ɫ (���ǻ��)��
��5������ȤС����ʵ���У�������Ͳ�в�û���ռ���Һ�壬����Ϊ����ʧ�ܵ�ԭ�������
____________________________________________________
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
��10�֣���ѧС��ͬѧ���ݻ�ѧ��ӦZn��2H2SO4(Ũ) ZnSO4��SO2����2H2O��ȡ22.4 L(��״��)SO2���塣ȡ65.0 gп����98%��ŨH2SO4(�ѣ�1.84 g��cm-3)110 mL��ַ�Ӧ��пȫ���ܽ⡣�����Ƶõ����壬��ͬѧ��Ϊ���ܻ���������Ϊ�ˣ���ѧС���ͬѧ���������ʵ��װ�ã�������ȡ���������̽����
ZnSO4��SO2����2H2O��ȡ22.4 L(��״��)SO2���塣ȡ65.0 gп����98%��ŨH2SO4(�ѣ�1.84 g��cm-3)110 mL��ַ�Ӧ��пȫ���ܽ⡣�����Ƶõ����壬��ͬѧ��Ϊ���ܻ���������Ϊ�ˣ���ѧС���ͬѧ���������ʵ��װ�ã�������ȡ���������̽����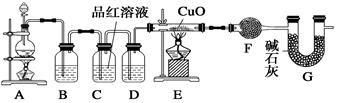
��ش��������⣺
��1��װ��A������Һ�����������Ϊ ��98%��ŨH2SO4(�ѣ�1.84 g/cm3)�����ʵ���Ũ���� ��
��2����д�����������ķ�Ӧ�����ӷ���ʽ ��
��3����װ��B��Ϊ������SO2������ѡ�������Լ��е� ������ţ���
A��NaOH��Һ B��ŨH2SO4 C��KMnO4��Һ
�ڿ�֤ʵһ������п����һ������Ũ���ᷴӦ�����ɵ������л���������ʵ�������� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
(18��) (1)ijʵ��С��ļ�ͬѧ��ͼl��ʾװ����ȡ������������(�����豸���г̶ֹ�װ�þ���ȥ)��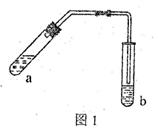
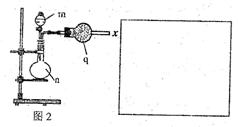

���Թ�a����Ҫ����Ũ���ᡢ��������Ҵ���2mL����ȷ�ļ���˳������ ��
���Թ�a�м������Ƭ�������� ���������һ��ʱ��������ǼӴ�Ƭ��Ӧ�ò�ȡ����ȷ������ (�����)��
| A���������� | B�����貹�� | C����ȴ�� | D���������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
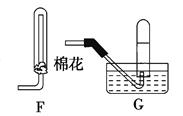
��18�֣�ʵ���ҳ�������װ���Ʊ����ռ������������壬��̽�������ʡ�
��1��װ��A�еķ�Һ©����ʢװ��Һ��ͨ���� ��Բ����ƿ��Ԥ�ȼ������ͭƬ�������ļ۸��ͭ�ļ۸�ͣ��˴���ͭƬ������Ƭ��ԭ���� ��
��2�������D��װ�ĸ��������ˮ�Ȼ��ƣ��������� ��
��3������ʱ������ƣ�Ũ������ϡ��װ��E������������β�������չ����з�����Ӧ�Ļ�ѧ����ʽ���£�2NO2��2NaOH��NaNO3��NaNO2��H2O ��NO2��NO��2NaOH��2NaNO2��H2O
��NO��NO2����������ɿɱ�ʾ��NOx���û������ͨ��NaOH��Һ����ȫ����ʱ��x��ֵ����Ϊ ������ĸ��
| A��1.1 | B��1.2 | C��1.5 | D��1.8 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
������������ɵ�һ����ȡSO2����֤���������ԡ���ԭ�Ժ�Ư���ԡ�(ͼ������̨�����е�ʡ�ԣ�װ�â���E��������������)�ڢܢ�װ���������Լ�ֻ�ܴ�����������ѡ�ã���ˮ��ŨH2SO4����ˮ�����ᡢƷ����Һ���ռ���Һ��ʯ��ˮ��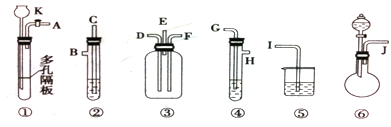
�Իش�
��1����������ʱ������������˳�������J��_____��_____��_____��H��D��______��I��______��______��
��2��װ�â����Լ���SO2�Ļ�ԭ�ԣ���ʢ�Լ������______��װ�â�����ʢ�Լ������______��װ�â�����ʢ�Լ������_______��Ŀ����
��3���������Ӻú��������װ�âٵ������ԣ���������Ҫ�� ��
��4����д��װ���з�Ӧ�Ļ�ѧ����ʽ ��
��д��װ���з�Ӧ�Ļ�ѧ����ʽ ��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com