
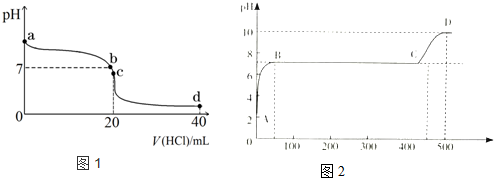
���� ��1��һˮ�ϰ���������ʣ���ˮ��Һ����ڵ���ƽ�⣻
��2��pH=7ʱ����Һ��cc��H+��=c��OH-������Һ�д��ڵ���غ㣬�ٽ�ϵ���غ��ж�c��Cl-����c��NH4+������Դ�С��
��3��c��ʱ������ǡ�÷�Ӧ�����Ȼ�泥��Ȼ����ǿ�������Σ�笠�����ˮ���ʹ����Һ�����ԣ�
��4��d��ʱ����Һ�е�����Ϊ�����ʵ���Ũ�ȵ��Ȼ�狀����ᣬ��Һ�����ԣ��Ȼ�����ȫ���룬笠�������ˮ�⣬�ݴ��ж���Һ������Ũ�ȴ�С��
��5����ͬ�����£���ĵ���ƽ�ⳣ��Խ�����������Խǿ��������ӵ�ˮ��̶�ԽС������Һ�ļ���Խ����
��6����ҺpH��7��˵����Һ�����ԣ����̼������Һ��Ӧ�����Ȼ��ƺ�ˮ��������̼����pH����ʱ��̼���ƺ��Ȼ��Ʒ������ֽⷴӦ��̼����Ϊǿ�������Σ�����Һ�ʼ��ԣ�����ˮ�����ӻ�������������������Ũ�ȣ�
��� �⣺��1��һˮ�ϰ���������ʣ���ˮ��Һ��ֻ�в��ֵ��룬��������������Ӻ�笠����ӣ�һˮ�ϰ��ĵ��뷽��ʽΪ��NH3•H2O?NH4++OH-��
�ʴ�Ϊ��NH3•H2O?NH4++OH-��
��2��b��ʱpH=7������Һ��cc��H+��=c��OH-������Һ�д��ڵ���غ㣬���ݵ���غ��c��Cl-��+c��OH-��=c��NH4+��+cc��H+�������Ե�c��Cl-��=c��NH4+�����ʴ�Ϊ��=��
��3��c��ʱ����ǡ�÷�Ӧ�����Ȼ�泥��Ȼ����ǿ�������Σ�笠�����ˮ���ʹ��Һ��������Ũ�ȴ�������������Ũ�ȣ�����Һ�����ԣ�ˮ�����ӷ���ʽΪ��NH4++H2O?NH3•H2O+H+��
�ʴ�Ϊ��NH4++H2O?NH3•H2O+H+��
��4��d��ʱ��������ʵ����ǰ�ˮ��2���������ʱ����Һ�е�����Ϊ�����ʵ���Ũ�ȵ��Ȼ�狀����ᣬ��Һ�����ԣ��Ȼ�����ȫ���룬笠�����ˮ�ˮ��̶Ƚ�С����������غ�֪����Һ������Ũ�ȴ�С˳����
c ��Cl-����c ��H+����c ��NH4+����c ��OH-����
�ʴ�Ϊ��c��Cl-����c��H+����c��NH4+����c��OH-����
��5����ͬ�����£���ĵ���ƽ�ⳣ��Խ�����������Խǿ��������ӵ�ˮ��̶�ԽС������Һ������������Ũ��ԽС��������Ũ��Խ���ݵ���ƽ�ⳣ��֪������ǿ��˳���ǣ��������������ӣ�̼�̼��������ӣ�����ˮ��ǿ��˳���ǣ�̼������ӣ�̼��������ӣ���������ӣ�����������ӣ�
��������ˮ��̶�֪�������������ˮ��̶���С��������Һ����������������Ũ���������������Ũ��������B��ˮ��̶���ǿ����C������ҺC�м�����ǿ������������Ũ�����
�ʴ�Ϊ��B��C��
��6����ҺpH��7��˵����Һ�����ԣ����̼������Һ��Ӧ�����Ȼ��ƺ�ˮ��������̼�����ӷ���ʽΪ��2H++CO32-=H2O+CO2������pH����ʱ��̼���ƺ��Ȼ��Ʒ������ֽⷴӦ�����ӷ���ʽΪCa2++CO32-=CaCO3����̼����Ϊǿ�������Σ�����Һ�ʼ��ԣ�D��ʱ�����Һ����ˮ���������c��OH-��=$\frac{1{0}^{-14}}{1{0}^{-10}}$mol/L=10-4 mol/L��
�ʴ�Ϊ��2H++CO32-=H2O+CO2����Ca2++CO32-=CaCO3����10-4��
���� ���⿼�����������Һ�����ж�����㣬��Ŀ�Ѷ��еȣ���ȷ��Һ�е����ʼ��������ǽⱾ��ؼ����ٽ����Һ������Լ������غ�͵���غ��������������������ѧ���ķ�����������ѧ����������


 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �����ʵ���NaHCO3��Na2CO3�ֱ���ͬŨ��������ȫ��Ӧ�����ĵ��������Na2CO3��NaHCO3�Ķ��� | |
| B�� | Na2O2��Na2O�����Ժ����ᷴӦ������Ӧ���Σ������ڼ��������� | |
| C�� | ������ʯ��ˮ�ֱ����NaHCO3��Na2CO3��������Һ�У�ֻ��Na2CO3��Һ�������� | |
| D�� | Na2O2��Na2O��Na2O���ȵ���Na2O2��һ�������¿���ת��ΪNa2O |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������

| �¶�/�� | 400 | 500 | 830 |
| ƽ�ⳣ��K | 10 | 9 | 1 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | KClO3��O2 | B�� | CO2��CO | C�� | Fe��Fe3O4 | D�� | CuO��CuSO4 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �Ȳ���ʹ¯��˲��������ֲ��ܽ�ʡȼ�� | |
| B�� | �䲻��ʹ¯��˲������������Խ�ʡȼ�� | |
| C�� | ����ʹ¯��˲������ֿ��Խ�ʡȼ�� | |
| D�� | ���ܽ�ʡȼ�ϣ�����ʹ¯��˲����� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� |  ����Cl2 | |
| B�� |  ����90ml 0.1mol•L-1������Һ | |
| C�� |  ����е����ϴ�Ļ���Һ������ | |
| D�� |  ���뻥�����ܵ�����Һ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
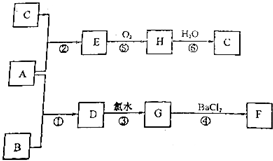
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | 39g���к���C�TC����Ϊ1.5NA | |
| B�� | 0.5molFeBr2���״����33.6L������Ӧʱת�Ƶ�����Ϊ3NA | |
| C�� | 1L0.5mol•L-1��NaClO��Һ�к��е�ClO-������Ϊ0.5NA | |
| D�� | ���³�ѹ�£�14g��C2H4��C3H6��ɵĻ�������к��е�ԭ������Ϊ3NA |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ���з�Ӧ�ﵽƽ��ʱ��Q1=Q | |
| B�� | �ﵽƽ�����C������������Ҵ� | |
| C�� | �ﵽƽ����������м���0.25molA��0.75molB��1.5molC��ƽ��������C�ķ����ƶ� | |
| D�� | ���е��Ȼ�ѧ��Ӧ����ʽΪ2C��g��?A��g��+3B��g����H=+Q2kJ/mol |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com