
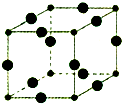
 Cu3N�������õĵ�ѧ��ѧ���ܣ��ڵ��ӹ�ҵ�����պ�������������ͨѶ�����Լ���ѧ��ҵ�������У������Ź㷺�ġ���������ľ����ã�
Cu3N�������õĵ�ѧ��ѧ���ܣ��ڵ��ӹ�ҵ�����պ�������������ͨѶ�����Լ���ѧ��ҵ�������У������Ź㷺�ġ���������ľ����ã�| 1 |
| 4 |
| 1 |
| 8 |
| m |
| V |
| 1 |
| 2 |
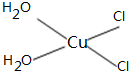 ���ʴ�Ϊ��
���ʴ�Ϊ�� ��
��| 1 |
| 4 |
| 1 |
| 8 |
| m |
| V |
| ||
| [(2a+2b)��10-10]3 |
| 103��1030 |
| 4(a+b)3NA |
| 103��1030 |
| 4(a+b)3NA |


 Ӧ�����������Ĵ���ѧ������ϵ�д�
Ӧ�����������Ĵ���ѧ������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
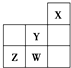 ������Ԫ��W��X��Y��Z��Ԫ�����ڱ��е�λ����ͼ��ʾ������˵���в���ȷ���ǣ�������
������Ԫ��W��X��Y��Z��Ԫ�����ڱ��е�λ����ͼ��ʾ������˵���в���ȷ���ǣ�������| A��W������������Ӧ��ˮ������ǿ�� |
| B��Y��ԭ�Ӱ뾶��ͬ��������Ԫ������С |
| C��W�ķǽ����Ա�Z��ǿ |
| D��Z����̬�⻯����ȶ�����ͬ����Ԫ������ǿ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A�������ʯ��ˮ��Ũ���� |
| B�����Ը��������Һ��Ũ���� |
| C����ˮ��Ũ���� |
| D��Ũ���ᡢ���Ը��������Һ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
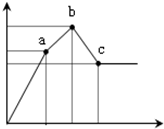 ��100mL0.1mol/L�������[NH4Al��SO4��2]��Һ����ε���0.1mol/L Ba��OH��2��Һ������Ba��OH��2��Һ���V�������꣩�ı仯�����������ʵ���n�ı仯��ͼ��ʾ��
��100mL0.1mol/L�������[NH4Al��SO4��2]��Һ����ε���0.1mol/L Ba��OH��2��Һ������Ba��OH��2��Һ���V�������꣩�ı仯�����������ʵ���n�ı仯��ͼ��ʾ���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
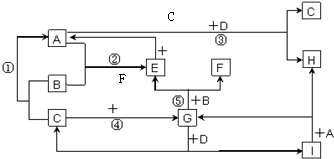
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A������AΪSO2��H2�Ļ���� |
| B����Ӧ�й�����Zn 65g |
| C������A��SO2��H2�������Ϊ1��4 |
| D����Ӧ�й�ת�Ƶ���2mol |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com