
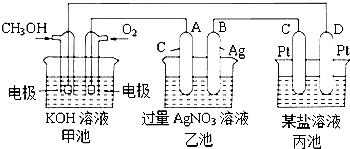
 ͼ��һ����ѧ���̵�ʾ��ͼ��
ͼ��һ����ѧ���̵�ʾ��ͼ������ ���и���Ϊ�״�������Ϊ������Ϊԭ��أ�������ӦΪCH3OH+8OH-6e-�TCO32-+6H2O����Ϊ���أ�A��ԭ��ص�������������AΪ����������4AgNO3+2H2O$\frac{\underline{\;���\;}}{\;}$4Ag+O2��+4HNO3������B������Ag�������������������õ����غ��֪��O2��4Ag��4HNO3������ij�缫����1.60gij�����������Ա�������ǿ�Ľ������Ӿ����ܣ��Դ������
��� �⣺��1����ͼ��֪����Ϊȼ�ϵ�أ���Ϊԭ��أ���Ϊ�ҵĵ�Դ������Ϊ���أ��ʴ�Ϊ�����أ�
��2��D��ԭ��صĸ�������������D�ǵ��ص���������C��Pt���缫���������������ʴ�Ϊ��������
��3��ͨ��CH3OH�ĵ缫Ϊ������ʧȥ���ӣ���缫��ӦʽΪCH3OH+8OH-6e-�TCO32-+6H2O���ʴ�Ϊ��CH3OH+8OH-6e-�TCO32-+6H2O��
��4����Ϊ���أ�A��ԭ��ص�������������AΪ��������Һ�������ӡ����������ӷŵ磬����4AgNO3+2H2O $\frac{\underline{\;���\;}}{\;}$4Ag+O2��+4HNO3�����ӷ�Ӧ����ʽΪ��4Ag++2H2O$\frac{\underline{\;���\;}}{\;}$4Ag+O2��+4H+��
�ʴ�Ϊ��4Ag++2H2O$\frac{\underline{\;���\;}}{\;}$4Ag+O2��+4H+��
��5���ɵ����غ��֪��O2��4Ag��4HNO3��n��O2��=$\frac{5.4g}{108g/mol}$��$\frac{1}{4}$=0.0125mol������µ����Ϊ0.0125mol��22.4L/mol=0.28L=280mL������ij�缫����1.60gij�����������Ա�������ǿ�Ľ������Ӿ����ܣ���BD���ϣ�
�ʴ�Ϊ��280��BD��
���� ���⿼��ԭ��غ͵��أ���ȷ�����ĵ缫��Ӧ�����ӵķŵ�˳�ɽ��ע������غ��ڼ����е�Ӧ�ã���Ŀ�Ѷ��еȣ�ע���˻���֪ʶ�Ŀ��飮


 �Ǽ�����������ϵ�д�
�Ǽ�����������ϵ�д� â���̸������Ծ�ϵ�д�
â���̸������Ծ�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ʯ�͵ķ����ú�ĸ����������仯 | |
| B�� | ���顢�����Ҵ������Է���ȡ����Ӧ | |
| C�� | ���ۺ���ά�ػ�Ϊͬ���칹�� | |
| D�� | ����ܷ���ˮ�ⷴӦ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
| �¶ȣ������¶ȣ� | K1 | K2 |
| 973 | 1.47 | 2.38 |
| 1173 | 2.15 | 1.67 |
| ʱ��/min | CO2 | H2 | CO | H2O |
| 0 | 0.2000 | 0.3000 | 0 | 0 |
| 2 | 0.1740 | 0.2740 | 0.0260 | 0.0260 |
| 3 | C1 | C2 | C3 | C3 |
| 4 | C1 | C2 | C3 | C3 |
| 5 | 0.0727 | 0.1727 | 0.1273 | 0.1273 |
| 6 | 0.0350 | 0.1350 | 0.1650 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
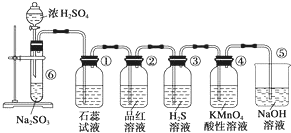 ͼ��ʵ������ȡSO2����֤SO2��ijЩ���ʵ�װ�ã��Իش�
ͼ��ʵ������ȡSO2����֤SO2��ijЩ���ʵ�װ�ã��Իش��鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | 1��1 | B�� | 2��5 | C�� | 5��2 | D�� | 2��3 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
| A�� | 100mL��Ͳ | B�� | ������ | C�� | ������ƽ | D�� | 50mL����ƿ | ||||
| E�� | 10mL��Ͳ | F�� | ��ͷ�ι� | G�� | 50mL�ձ� | H�� | 100mL����ƿ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ������ĵ��볣����Ki1��H2CO3����Ki��HX����Ki��HY����Ki2��H2CO3�� | |
| B�� | NaX��Һ��ͨ������CO2�������Ũ�ȣ�c��HCO3-����c��CO32-����c��X-�� | |
| C�� | ͬŨ����Һ�ļ��ԣ�NaX��Na2CO3��NaY��NaHCO3 | |
| D�� | ���H+��������Y-��CO32-��X-��HCO3- |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com