
ij���Ը���Ϊԭ�����ǣ��Բ����Ĵ�������������ͼ��ʾת�������ۺ����á�����B��Aˮ������ղ��C�Ļ�ѧʽΪC3H6O3��һ��������2��C���Ӽ���ȥ2��ˮ���ӿ�����һ����Ԫ��״�����D��ʹ��ˮ��ɫ��F�Ǿ�����ζ��Һ�塣(ͼ�в��ַ�Ӧ����������û���г�)
�ش��������⣺
(1)A��������________��F�Ľṹ��ʽ__________��
D�Ľṹ��ʽ________��
(2)C��D�ķ�Ӧ����Ϊ________��D��E�ķ�Ӧ����Ϊ________��
(3)д�����з�Ӧ�ķ���ʽ��
A��B��______________________________��
G��H��______________________________________��
(4)H�������������ŵ�������________��ʵ�����г����ڼ���ù����ŵ��Լ���������(ֻдһ��)_____________________________��

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
���������в��������ᷴӦ����
| A��Na | B��CH3CHO | C��Cu(OH)2 | D��Na2CO3 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��5�֣���֪±������R-X���ڼ��������¿�ˮ��õ�����R-OH�����磺CH3CH2-X+H2O  CH3CH2-OH+HR����������ת����ϵ:
CH3CH2-OH+HR����������ת����ϵ: 
�ش��������⣺
��1����Ӧ1���Լ�������Ϊ __________��X�Ľṹ��ʽΪ______��Y�Ľṹ��ʽΪ______��
��2��д����Ӧ3�ķ���ʽ___________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��Ҫ������������⣺
��ij�л���ļ���ʽ�� ����д������NaOH��Һ��Ӧ�Ļ�ѧ����ʽ ��
����д������NaOH��Һ��Ӧ�Ļ�ѧ����ʽ ��
��DDT����ϳɵĵ�һ���л���ũҩ������ӽṹ���ģ����ͼ��ʾ������������ʵĺ˴Ź���1H��ͼ���� �����շ塣
��F��G�� �� ���ճ������г��õ����ֺϳɸ߷��Ӳ��ϣ�����ij�������з�Ӧ�õ���
�� ���ճ������г��õ����ֺϳɸ߷��Ӳ��ϣ�����ij�������з�Ӧ�õ���
��ش��������⣺
��F�Ľṹ��ʽΪ ��
��C�����������ŵ�����Ϊ ���� �ǣ����ԲⶨD�����������š�
��A��B�Ļ�ѧ����ʽΪ ��
����֪2RCH(OH)COOH 2H2O +
2H2O + 
��ο���Ŀ�еĺϳ�;�������ϳ� ����ʼԭ�ϵ�ij���Ľṹ��ʽΪ �������� ����Ӧ�����Ժϳɲ��
����ʼԭ�ϵ�ij���Ľṹ��ʽΪ �������� ����Ӧ�����Ժϳɲ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
һ���л���Ľṹ��ʽΪ��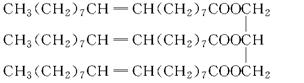
�Իش��������⣺
(1)�û������������________��
A��ϩ�� B���� C����֬ D���߷��ӻ�����
(2)�û�������ܶ�________��
A����ˮ�� B����ˮС
(3)�û����ﳣ���µ�״̬Ϊ________��
A��Һ�塡��B�����塡��C������
(4)��������ܷ�Ӧ����________��
A��NaOH��Һ B����ˮ
C���Ҵ������� D�����ᡡ���� E��H2
д�����л�����ѡ���Ļ����ﷴӦ�Ļ�ѧ����ʽ(��дһ��)��________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��ͼΪӲ֬��������ڼ���������ˮ���װ�á�����������Ӧʱ�IJ������£�
(1)��Բ����ƿ��װ��7��8 gӲ֬���������Ȼ�����2��3 g�������ơ�5 mLˮ��10 mL�ƾ�������ƾ�������Ϊ ��
(2)����ʯ��������Ӧ��������Լ10 min��������Ӧ������ɣ����õĻ����Ϊ (�����Һ����������Һ��������Һ�����塱)��
(3)�����û�����м��� (�ѧʽ)������һ��ʱ�䣬��Һ��Ϊ�������㣬������ �㣬���������Ϊ ��
(4)ͼ�г��������ܵ�����Ϊ ��
(5)д���÷�Ӧ�Ļ�ѧ����ʽ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
ͨ����ʳ���Ϳɻ��ij�����л�������X,����Է�������Ϊ46,����̼����������Ϊ52.2%,�����������Ϊ13.0%��
(1)X�ķ���ʽ������������
(2)X������Ʒ�Ӧ�ų�����,��Ӧ�Ļ�ѧ����ʽ������������
(3)X������е�������ͭ�������·�Ӧ����Y,Y�Ľṹ��ʽ������������
(4)X�����Ը��������Һ��Ӧ������Z���ڼ��Ⱥ�Ũ����������,X��Z��Ӧ������һ������ζ������W,��184 g X��120 g Z��Ӧ������106 g W,����÷�Ӧ�IJ���(Ҫ��д���������)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��ͼ��ʾ������ˮ��ɲ���ij�л�������A��A�ڲ�ͬ�������������£���������B(C6H12O7)��C(C6H10O8)��B��C�����ܷ���������Ӧ��A��B��C�����Ա�ǿ��ԭ����ԭ��ΪD(C6H14O6)��B��ˮ�ɵ���Ԫ�����������E����Ԫ�����������F��
��֪��������ʱ����������״����ǣ�RCHO���ף�R��CH2OH��֮�� ���ѡ�
���ѡ�
�������пո�����дA��B��C��D��E��F�Ľṹ��ʽ��
A��____________________��B.____________________��
C��____________________��D.____________________��
E��____________________��F.____________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�����ӵĹ�ҵ��ˮ�Ĵ���������ͼ��ʾ��
��1��������ͼ�豸���н��е���______��������д�������ƣ���
��ͼ�У���ѭ��ʹ�õ������ǣ�C6H6��CO2______��______��
��2��Ϊ�˷�ֹˮԴ��Ⱦ���ü����������Եķ�������ij�����ŷŵ���ˮ�����ޱ��ӣ��˷�����_______________________________________________________��
��3���ӷ�ˮ�л��ձ��ӵķ����ǣ������л��ܼ���ȡ��Һ�еı��ӣ��ڼ���ij��ҩƷ��ˮ��Һʹ�������л��ܼ����룻��ͨ��ij�������������ӡ���д���ڡ��������ķ�Ӧ����ʽ��
_________________________________________________________________________________________��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com