
����Ŀ������(P4)��һ�ֳ����ľ���,�������Ʊ��ϴ������ᡣ
��1��������________(��ԭ�ӻ����)���壬31 g������������������ȫȼ���ͷų�745.5 kJ����������д������ȼ�յ��Ȼ�ѧ��Ӧ����ʽ��______________________________________��
��2����֪����������Һ�ɷ������·�Ӧ��________P4+___HClO3+___ ______��____HCl+____H3PO4,��ƽ�����������Ӧ����ʽ______________���÷�Ӧ����������___________��
��3�������ж���ʵ���ҿɲ���CuSO4��Һ���д������䷴ӦΪ��11P4��60CuSO4��96H2O==20Cu3P��24H3PO4��60H2SO4���÷�Ӧ������������________������11 mol P4��Ӧ������________ mol����ת�ơ�
���𰸡����� P4(s) + 5O2(g) = 2P2O5(s) ��H= -2982.0kJ/mol 3 10 18 H2O 10 12 HClO3 H3PO4 120
��������
��1������Ϊ���Ӿ��壬�����ʽΪP4��31g������������������ȫȼ������P2O5���壬�ͷų�745.5kJ����������1mol������ȫȼ�շų�������=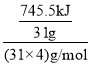 =2982kJ��������ȼ�յ��Ȼ�ѧ��Ӧ����ʽΪ��P4��s��+5O2��g��=2P2O5��s������H=-2982 kJ/mol��
=2982kJ��������ȼ�յ��Ȼ�ѧ��Ӧ����ʽΪ��P4��s��+5O2��g��=2P2O5��s������H=-2982 kJ/mol��
�ʴ�Ϊ�����ӣ�P4��s��+5O2��g��=2P2O5��s������H=-2982 kJ/mol��
��2����Ӧ��PԪ�صĻ��ϼ���0�����ߵ�+5�ۣ�P4Ϊ��ԭ����ClԪ�صĻ��ϼ���+5�۽��͵�-1�ۣ�HClO3Ϊ�����������ݵ�ʧ������Ŀ��ȿ�֪��������֮��Ϊ3��10�����������غ㶨�ɿ�֪ƽ���Ļ�ѧ����ʽΪ3P4+10HClO3+18H2O=10HCl+12H3PO4��
�ʴ�Ϊ��3��10��18H2O��10��12��HClO3
��3���÷�Ӧ�У�CuԪ�صĻ��ϼ���+2�۽��͵�+1�ۣ�������Ԫ����0�۽��͵�-3�ۣ�������Ԫ����0�����ߵ�+5�ۣ���������������H3PO4���÷�Ӧ����11mol���ײμӷ�Ӧ����ת�Ƶ��ӵ����ʵ���=24����5-0��mol=120mol��
�ʴ�Ϊ��H3PO4��120��


 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ԭ����SCN���ϼ�Ϊ��1�ۣ��ںܶ���±��ԭ�����ƣ���˳�Ϊ����±��������±��������±���ӻ�ԭ��ǿ��˳��ΪCl����Br����SCN����I������֪������I2��S�����з�Ӧ����ȷ����(����)
A. (SCN)2��2Br��=Br2��2SCN��
B. (SCN)2��H2S=2H����2SCN����S��
C. 4H����2SCN����MnO2![]() Mn2����(SCN)2����2H2O
Mn2����(SCN)2����2H2O
D. (SCN)2��2I��=2SCN����I2
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����2molNaHCO3��һ������Na2O2�����ϣ��ڼ��������������ַ�Ӧ�����ù�����вⶨ������Na2O2��ʣ�࣬�����ù�������ʵ�����n��Ϊ
A.1mol��n��2molB.1mol��n��4mol
C.2mol��n��4molD.1mol��n��4mol
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���������Ҫ��MoS2��������Ca��Si��Cu��Zn��Fe��Ԫ�ء����û�����Ʊ�������淋���һ�����������������ͼ��ʾ��
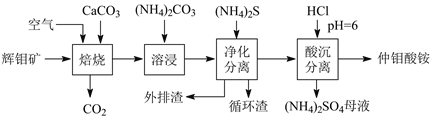
�ش��������⣺
��1�������¶�Ϊ400�棬MoS2ת��ΪCaMoO4��CaSO4����Ӧ��ÿĦMoS2ת�Ƶĵ�����Ϊ_________��������泥�������泥��е���������Mo7O24n-����n��_______��
��2����ͳ��������650���£�ʹMoS2ֱ��������е�O2��Ӧ����MoO3��SO2��ͼʾ����������ռ���CaCO3���ŵ���______________��
��3���ܽ�ʱ��CaMoO4�������ֽⷴӦ�Ļ�ѧ����ʽ��___________��ѭ��������Ҫ�ɷ���CaCO3������������Ҫ��________��Cu��Zn��Fe�����
��4����֪��������Ksp(CaCO3)��2.8��10-9��Ksp(CaSO4)��9.1��10-6����(NH4)2SO4ĸҺ������ѭ������CaCO3��������ʹ�����ת��Ϊ̼��泥������ܽ�ѭ��ʹ�ã���ԭ����_______��
��5���������ֽ��⾫��ʱ���ö��Ե缫����⾫���NaCl�Ļ�Ͻ�Һ��������Ĥ��������ҺpH��9������������Ϊ________����Ͻ�Һ�У���������ת�����ɵ�NaClO����MoS2����MoO42-��SO42-�����ӷ���ʽ��______________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��Mg-VOCl2�����һ�ֻ��������Ӵ��������Ͷ��ε�أ����װ��ʾ��ͼ���¡��ܷ�ӦΪMg��2VOCl2![]() MgCl2��2VOCl������˵���������
MgCl2��2VOCl������˵���������

A.����Mg���缫���ϱ�Li�İ�ȫ�Ը���
B.Ϊ�õ�س��ʱMg�缫Ӧ���Դ����������
C.�ŵ�ʱ������ӦΪVOCl2��e��= VOCl��Cl��
D.�ŵ������Cl��������������Һ����Mg�ƶ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����NA��ʾ�����ӵ�������ֵ������������ȷ���ǣ� ��
A.1mol NH4+�к��еĵ�����Ϊ11 NA
B.0.1mol��L-1��Ba(OH)2��Һ�к��е�OH- ��ĿΪ0.2NA
C.0.1NA���ȷ�������1Lˮ�У�������Һ��c(C1-)=0.1mol��L-1
D.1mol NH3����ˮ�����1L��Һ�����ð�ˮ�����ʵ���Ũ��Ϊ1mol��L-1
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���״���һ�ֳ�����ȼ�ϣ�Ҳ����Ҫ�Ļ�������ԭ�ϡ��ش��������⣺
��1�����ü״����ఱ�������Ƶö��װ���
��֪��2CH3OH(g)��3O2(g) 2CO2(g)��4H2O(g) H1��-3122kJ/mol
4NH3(g)��3O2(g) 2N2(g)��6H2O(g) H2��-472 kJ/mol
4(CH3)2NH(g)��15O2(g) 8CO2(g)��14H2O(g)��2N2(g) H3��-7492 kJ/mol
���Ʊ����װ���Ӧ2CH3OH(g)��NH3(g) (CH3)2NH(g)��2H2O(g)��H��_____ kJ/mol��
��2��һ�������£��״����ఱ����ԭ������c(CH3OH):c(NH3)�ֱ�Ϊ1:1��2:1��3:1ʱ��NH3��ƽ��ת�������¶ȱ仯�Ĺ�ϵ��ͼ��
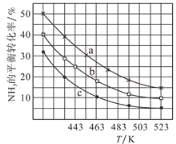
�ٴ���ԭ������c(CH3OH):c(NH3)��1:1��������________��
��һ���¶��£�����ԭ������c(CH3OH):c(NH3)��3:1��������NH3ƽ��ת���ʵĴ�ʩ��_______��
���¶�Ϊ443Kʱ����c(CH3OH):c(NH3)��2:1Ͷ�ϣ���NH3����ʼŨ��Ϊ2mol/L����Ӧ�ﵽƽ��ʱ��(CH3)2NH���������Ϊ_______�����¶��µĻ�ѧƽ�ⳣ��Ϊ________ ��
��3���״���ͨ���绯ѧ�����ɼ���ֱ���Ƶã�װ������ͼ��ʾ���������CH3OH�Ĺ��̷�Ϊ3����

��ͨ��ʱ����������ת��Ϊ���Ե�ԭ���ȣ�Cl������
��Cl���������ڵ缫�ϵ�CH4��Ӧ����HCl��CH3Cl��
���ڼ��Ե��Һ�У�CH3Clת��ΪĿ�����CH3OH��
����ٵĵ缫��ӦʽΪ__________������۵����ӷ���ʽΪ__________��ά�ֵ���ǿ��Ϊ1.5A��װ�ù���2Сʱ�������Ͽ��Ƶ�CH3OH������Ϊ________g������֪F��96500C/mol�������������ܽ�����أ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ͼ��ʾת����ϵ���漰�������ʾ�������Ԫ����ɣ����ֲ�������ȥ��������C��DΪ���ʣ�A��B��E��F��GΪ�������C��D�ķ�Ӧ�⣬������Ӧ������Һ�н��С�����д���пհס�
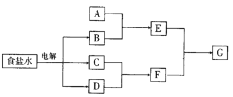
��1�� ��A�dz����Ľ���������������F����ʱ��GΪ�������Σ���A�Ļ�ѧʽΪ_________��_________��
��2�� ��A��һ�ֳ������������F�������GΪͬһ�����������ʣ���A��������________������������_______��A����;��_________��
��3�� ��A��һ�־���Ư���Ե���̬�����A��___����A��һ����ζ����̬�����A�ĵ���ʽΪ______��
��4�� ��AΪ�л���������A��GΪͬһ���ʣ���д������A����ͬ������ʣ��Ľṹ��ʽ��_____��_____��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����Ҫ����գ�
��1���˶�Ա�����˷ܼ������������������ʱ�з�����ij���˷ܼ��Ľṹ��ͼ���ش��������⡣
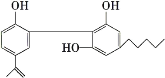
�����Ļ�ѧʽΪ____��
�ڴӽṹ�Ͽ���������___�ࡣ
���������Ĺ����ŵ�������___��
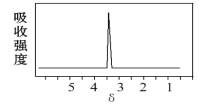
��2��������A��B�ķ���ʽ����C2H4Br2��A�ĺ˴Ź�������ͼ��ͼ����A������Ϊ___����Ԥ��B�ĺ˴Ź�����������___����(�ź�)��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com