
��֪ij����Һ��Ag+��Fe2+��Al3+��K+��Ba2+��NH4+��NO3����SO42���е�������������ɣ���������ʵ�飺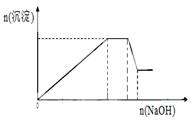
��һ�������������ϡ���ᣬ�������ɡ�
�ڶ������������������ϡ���ᣬ�а�ɫ�������ɡ�
�����������ˣ�ȡ������Һ������NaOH��Һ����Һ�ʼ��ԣ�
�ڴ˹�������Һ�������ı仯����ͼ��ʾ�����ȸ���Һ��
�ɲ���ʹʪ��ĺ�ɫʯ����ֽ����ɫ�����塣
��������ʵ������ش��������⣺
��1���ô���Һ��һ������ ���ӣ�һ��û�� ���ӣ������� ���ӡ�
��2��ijͬѧ���ò�pH�ķ������жϵ���NaOH��Һ���Ƿ�ʹ��Һ�ʼ��ԣ���ʵ������� ��
��3���������в���ʹʪ���ɫʯ����ֽ����ɫ����������ӷ���ʽΪ ���ò�����Ԥ
�ڻ��������һ�������ʵ�����������Ӧ�Ļ�ѧ����ʽΪ ��
��1��Fe2+��Al3+��Ba2+��NH4+��NO3- SO42-��Ag+ K+
��2��ȡһƬpH��ֽ���ڱ������ϣ��ýྻ�IJ�����պȡ����Һ������pH��ֽ���в�������Ӻ������ɫ�����գ��ж���Һ�Ƿ�ʼ��ԡ�
��3��NH4++OH-  NH3��+H2O 4Fe(OH)2+O2+2H2O=4Fe(OH)3
NH3��+H2O 4Fe(OH)2+O2+2H2O=4Fe(OH)3
���������������1������HCl��������˵��������Ag+���������������ϡ���ᣬ�а�ɫ��������˵������Ba2+���ӡ������ij�����Ӧ�ķ���ʽΪBa2++ SO42��=BaSO4��������˺����Һ�е���NaOH��Һ����Һ�ʼ������Ȳ����������������ﵽ���ֵ���ٵμӡ����������䣬������ּ���һ���֡�˵��������Fe2+��Al3+��NH4+��������Һ�ʵ����Կ�֪��Һ�л�Ӧ�ú��������ӡ�����Ba2+�� SO42�����ܹ��棬����Ba2+���ӣ�SO42�����ܴ��ڡ���һ��������NO3�����ӡ��ʸô���Һ��һ������Fe2+��Al3+��Ba2+��NH4+��NO3-��һ��������SO42-��Ag+�� K+������Ҳ����û�С���2���ж���Һ�Ƿ�ʼ��ԣ���ʵ�������ȡһƬpH��ֽ���ڱ������ϣ��ýྻ�IJ�����պȡ����Һ������pH��ֽ���в�������Ӻ������ɫ�����գ��ж���Һ�Ƿ�ʼ��ԡ���3������ʹʪ���ɫʯ����ֽ����ɫ����������ӷ���ʽΪNH4++OH-  NH3��+H2O . �ò����п��ܻ��������һ�������ʵ���������Fe(OH)2���ȶ������ױ������е���������ΪFe(OH)3���ῴ�����Ȳ�����ɫ��������������Ѹ�ٱ�Ϊ����ɫ������Ϊ���ɫ���������Ӧ�Ļ�ѧ����ʽΪ4Fe(OH)2 +O2+ 2H2O=4Fe(OH)3��
NH3��+H2O . �ò����п��ܻ��������һ�������ʵ���������Fe(OH)2���ȶ������ױ������е���������ΪFe(OH)3���ῴ�����Ȳ�����ɫ��������������Ѹ�ٱ�Ϊ����ɫ������Ϊ���ɫ���������Ӧ�Ļ�ѧ����ʽΪ4Fe(OH)2 +O2+ 2H2O=4Fe(OH)3��
���㣺�������ӵļ��������鼰��ѧ����ʽ�����ӷ���ʽ����д��֪ʶ��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
ij�ִ����к�����ɳ��Ca2����Mg2����Fe3����SO�����ʡ�ijͬѧ��ʵ����������������ִ����Ʊ����εķ�������(���ڳ������Լ��Թ���)��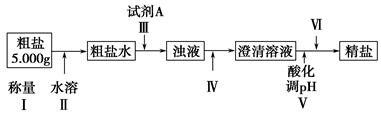
��ش��������⣺
(1)Ϊ������ѡ����������(�ñ����ĸ��д)��________��
A���ձ���B���Թܡ�C����������D����Һ©����E��©����F���ƾ��ơ�
G��������
(2)�������г���Na2CO3��Һ��NaOH��Һ��BaCl2��Һ��Ϊ�����Լ������������Լ���˳��Ϊ��NaOH��Һ��________��________��
(3)�������У��жϼ���BaCl2�ѹ����ķ�����___________________________________
(4)������Ӧѡ�������________����������������������Ⱥ�˳��Ե��������ʵ����������Ӱ����___________________________________
(5)��������________(ѡ��������������ƣ��ñ����ĸ�������Ⱥ�˳����д)��
a�����ˡ�ϴ�� B��������Ũ�� c����ȡ����Һ D����ȴ���ᾧ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
������أ� ������ǿ�����ԣ�������ԭΪ����أ���80 �����������ֽ⡣ʵ����ģ�ҵ�ϳɹ�����ص��������£�
������ǿ�����ԣ�������ԭΪ����أ���80 �����������ֽ⡣ʵ����ģ�ҵ�ϳɹ�����ص��������£�
��1������狀��������Ƴɵ��Һ���Բ����缫���е�⣬���ɹ��������Һ��д�����ʱ������Ӧ�����ӷ���ʽ_____________________________________
___________________________________��
��2����֪������ʵ��ܽ����������ͼ��ʾ����ʵ�������ᴿ������شֲ�Ʒ��ʵ������������Ϊ����������شֲ�Ʒ��������ˮ�У�________________�����
��3����Ʒ�й�����صĺ������õ��������вⶨ��ʵ�鲽�����£�
����1����ȡ���������Ʒ0.300 0 g�ڵ���ƿ�У�����30 mLˮ�ܽ⡣
����2������Һ�м���4.00 0 g KI���壨�Թ�������ҡ�ȣ��ڰ�������30 min��
����3���ڵ���ƿ�м�������������Һ�ữ���Ե�����Һ��ָʾ������0.100 0 mol��L��1Na2S2O3����Һ�ζ����յ㣬������Na2S2O3����Һ21.00 mL��
����֪��Ӧ��I2��2S2O32-=2I����S4O62-��
��������2��δ������ƿ���ڰ�������30 min�����������в���3����ⶨ�Ľ������________��ѡ�ƫ����ƫС��������Ӱ�족������������3�еζ��յ��������____________________________________________��
�ڸ�����������ɼ��������Ʒ�й�����ص���������Ϊ_______________��
��Ϊȷ��ʵ������ȷ�ԣ�����Ϊ����Ҫ____________________________��
��4����0.40 mol���������0.20 mol�������Ƴ�1 L��Һ����80 �������¼��Ȳ���tʱ������Һ�еμ�������FeCl3��Һ���ⶨ��Һ�и��ɷֵ�Ũ����ͼ��ʾ��H��Ũ��δ��������ͼ������X�Ļ�ѧʽΪ________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��������������������С������������������ˮ��Դ�ḻ���ش��������⣺
��1����ˮ����Ҫ���� �����ӣ�д���ӷ��ţ����ٴ��4�֣���
��2����ˮ������һ����Ч��ȥ�������������SO2�ķ������乤����������ͼ��ʾ��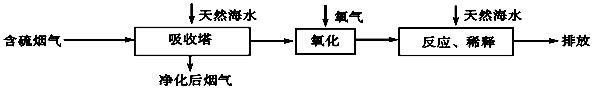
��Ȼ��ˮ�����˺���������Ҫ��O2���������������䷴Ӧ�Ļ�ѧ����ʽ�ǣ� ��������ġ���ˮ����Ҫ�ô�������Ȼ��ˮ��֮��Ϻ�����ŷţ��ò�������ҪĿ���� ��
��3����ͼ�Ǻ�ˮ�ۺ����õ�һ�����档
��ش��������⣺
I.�ٲ���Ҫ��Ӧ�����ӷ���ʽ�� ��
�弰�仯�������;�кܶ࣬д�����е�һ�� ��
II���ڢ۲���Ҫ���� ����ǰ������������þ�IJ�����Ϊ ����þ���ڶ�����̼��ȼ�յIJ���Ϊ ��
III������ˮ�Ժ��п����Ե�CaCl2��MgCl2��Na2SO4�����ʣ�ͨ�����¼���ʵ�鲽�裬���Ƶô�����ʳ��ˮ���� �����Թ�����Na2CO3��Һ���� �����Թ�����NaOH��Һ���� �����Թ�����BaCl2 ��Һ���ܵ���ϡ�����������ݲ������� ���� ��ȷ�IJ���˳����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��ѧʵ���Ҳ����ķ�Һ�к��д�������Ⱦ���������ʣ�Ϊ�˱�����������Щ��Һ���뾭����������ŷš�ij��ѧʵ���Ҳ����ķ�Һ�к������ֽ������ӣ�Fe3+��Cu2+����ѧС�����������ͼ��ʾ�ķ����Է�Һ���д������Ի��ս���������������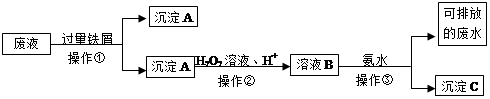
��1�������ٵ������� ��
��2������A�к��еĽ��������� ��
��3�����������������·�����Ӧ�����ӷ���ʽ ��
��4��������ҺB�к��еĽ��������ӳ��õ��Լ��� ��
��5���������з�����Ӧ�����ӷ���ʽΪ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
���������û�������������������ų��ķ�������Ҫ��ѧ�ɷ�ΪSiO2Լ45%��Fe2O3Լ40%��Al2O3Լ10%��MgOԼ5%��Ŀǰ�ҹ��Ѿ��ڼ�����ȡ��ͻ�ơ������������з�������ֳɷֲ��������á������̺�����������£�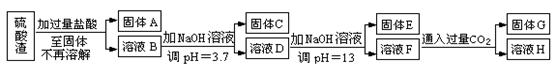
�����ϵ�֪��
| �������� | �ܶȻ�(Ksp) | pHֵ | |
| ��ʼ���� | ��ȫ���� | ||
| Mg(OH)2 | 5.6��10��12 | 9.3 | 10.8 |
| Fe(OH)3 | 2.8��10��16 | 2.7 | 3.7 |
| Al(OH)3 | 1.3��10��33 | 3.7 | 4.7 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��ϼʯ�ң���ѧʽΪ KNa3[AlSiO4]4����Ҫ�ɷ�Na2O��K2O��Al2O3��SiO2����̼���ơ�̼��غ��������Ĺ����������£�
��֪��NaHCO3��Һ��pHԼΪ8��9��Na2CO3��Һ��pHԼΪ11��12���ܽ���˹����������Һ�к��ơ��غ����Ŀ��������࣬�ƺ����������������ϼʯ���С��������ʵ��ܽ�ȼ���ͼ��
�Իش��������⣺
��1�����յõ�����M�Ļ�ѧ����ʽ��________________________________��
��2��X������___________����ҺW����Ҫ���е�������____________����д���֣�
��3��������õ�̼���ƾ���IJ���Ϊ���� �� �� ��ϴ�ӡ����
��4��̼�ữ���з�����Ҫ��Ӧ�����ӷ���ʽ��__________________________��
��5��̼�ữ�����pH��8��Ŀ����_______________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��ҵ������п����Ҫ�ɷ�ΪZnS��������Fe2O3�����ʣ�Ϊԭ������ZnSO4��7H2O�Ĺ����������£�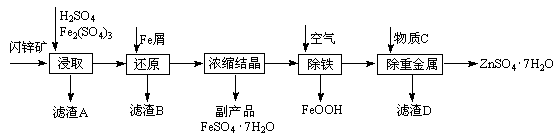
��1��������A�пɻ��һ�ֵ���ɫ�ǽ������ʵĸ���Ʒ���仯ѧʽΪ ��
��2����ȡ������Fe2(SO4)3�������� ����ȡʱFe2(SO4)3��ZnS������Ӧ�Ļ�ѧ����ʽΪ ��
��3���������̿�����Һ��pH��5.4���ң��÷�Ӧ�����ӷ���ʽΪ ���ù����ڿ�����ڴ������һ��������ԡ��ͷ��װ�ã���Ŀ���� ��
��4���û������ؽ���������������CΪ ��
��5������п���ܽ�����¶�֮��Ĺ�ϵ���±���
| �¶�/�� | 0 | 20 | 40 | 60 | 80 | 100 |
| �ܽ��/g | 41.8 | 54.1 | 70.4 | 74.8 | 67.2 | 60.5 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��һ�����ĩ,���п��ܺ���Na2CO3��NaCl��Na2SO4��Ba(NO3)2��K2CO3��K2SO4�е�һ�ֻ��֣��ְ����в������ʵ�顣
��1�����÷�ĩ����ˮ����ɫ��Һ�Ͱ�ɫ������
��2�����˳��ij����м���ϡ���ᣬ�в��ֳ����ܽ⣬ͬʱ������ɫ���塣
��3��ȡ��Һ����ɫ��Ӧ����֤����Һ�к�Na+������K+��
�����������ƶϣ�
��1���û������һ������ ���� ��һ�������� �����ܺ��� ��
��2����Ҫ�Կ��ܺ��е����ʽ��м��飬��β�����
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com