
��ҵ�Ͽ���ʳ�κ�ʯ��ʯΪ��Ҫԭ�ϣ�����ͬ�ķ������������ش��������⣺
��1��¬����������ʳ�Ρ�ʯ��ʯ��Ũ���ᡢ��̿Ϊԭ�ϣ��ڸ����½������գ��ٽ�ȡ���ᾧ���Ƶô��
��ʳ�κ�Ũ���ᷴӦ�Ļ�ѧ����ʽΪ___________��
�������ƺͽ�̿��ʯ��ʯ��Ӧ�Ļ�ѧ����ʽΪ_________����֪����֮һΪCaS����
��2������Ĺ�������ͼ��ʾ���õ���̼�����ƾ��������ɴ��
| | |
| |  |

��1����2NaCl+H2SO4(Ũ) 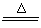 Na2SO4+2HCl��
Na2SO4+2HCl��
��Na2SO4+4C+CaCO3 CaS+Na2CO3+4CO��
CaS+Na2CO3+4CO��
Na2SO4+2C+CaCO3 CaS+Na2CO3+2CO2
CaS+Na2CO3+2CO2
��2����Ca��OH��2 NH3
��NH3+CO2+NaCl+H2O=NaHCO3��+NH4Cl
��3�������ԭ�ϵ������ʣ����ٷ�����CaCl2�����ŷţ������˰�����ŵ㣬����������ȱ�㣬ʹʳ�ε���������ߣ�NH4Cl �������ʣ�����ϳɰ������ϣ�ʹ�ϳɰ���ԭ���� CO ת���� CO2������ CaCO3�� CO2��һ����
��4�������У���ΪKHCO3��NH4Cl���ܽ�������¶ȸ���40��ʱ��KHCO3���ܽ�ȴ���NH4Cl�����½ᾧʱ�������϶��KCl��
����


 ����С����ͬ������ϵ�д�
����С����ͬ������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
ͬһ���Ʊ�װ�ÿ�������ȡ��ͬ�����壬��ֻ����ͼװ����ȡ���壬��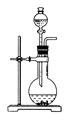
(1)�����±��ո�����������ҩƷ��
| �������� | ҩƷ | |
| ��Һ©���� | ��ƿ�� | |
| O2 | | MnO2 |
| H2 | ϡH2SO4 | |
| NH3 | | CaO |
| NO | ϡHNO3 | Cu |
| CO2 | ϡH2SO4 | ʯ��ʯ |
| C2H4 | C2H5OH | ϡH2SO4 |
| C2H2 | | CaC2 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
ˮ�ܿ��г�SiO2�⣬����9.24% CoO��2.78% Fe2O3��0.96% MgO��0.084 % CaO��������ȡ�ܵ���Ҫ�����������£�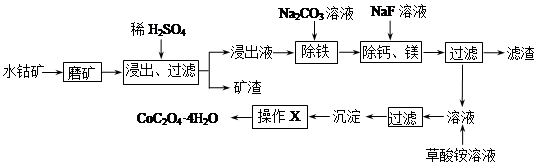
��1����һ��Ũ�ȵ�H2SO4��Һ�У��ܵĽ�������ʱ�䡢�¶ȵı仯��ͼ��ʾ�����������ɱ���Ч�ʣ���ѵĽ���ʱ��Ϊ Сʱ����ѵĽ����¶�Ϊ �档
��2������ƽ���г����Ļ�ѧ����ʽ��
Fe2(SO4)3+ H2O+ Na2CO3= Na2Fe6(SO4)4(OH)12��+ Na2SO4+ CO2��
��3�������ơ�þ����ԭ����ӦΪ��MgSO4+2NaF=MgF2��+Na2SO4 ��CaSO4+2NaF=CaF2��+Na2SO4
��֪Ksp��CaF2��=1.11��10��10��Ksp��MgF2��=7.40��10��11���������NaF��Һ��Ӧ��ȫ����ˣ�����Һ�� ��
��
��4�����������к�����������Ҫ��SO42����F���� �� ��������X������ �� ��
��5��ij����ӵ��������LiCoO2����Li+�������Ϊ����ʡ����ʱ��Li����ԭΪLi������ԭ����ʽǶ���ظ�������̼-6��C6���У���ͼ��ʾ������ط�ӦΪLiCoO2+C6  CoO2+LiC6��д���õ�طŵ�ʱ��������Ӧʽ ��
CoO2+LiC6��д���õ�طŵ�ʱ��������Ӧʽ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��ѧС���������������������װ��(����ͼ)���û������Ʊ�����ϩ��
��֪��
| | �ܶ�(g/cm3) | �۵�(��) | �е�(��) | �ܽ��� |
| ������ | 0.96 | 25 | 161 | ������ˮ |
| ����ϩ | 0.81 | ��103 | 83 | ������ˮ |


�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��ʴ���
��14�֣���������ƣ�Na2S2O3���׳Ʊ��շۣ�����������ҵ����Ӱ����Ҳ������ֽ��Ư�������ȼ��ȡ�ʵ���ҿ�ͨ�����·�Ӧ��ȡ��2Na2S��Na2CO3��4SO2��3Na2S2O3��CO2��

ͼ1 ͼ2
��1����ͼ1��ʾװ����ȡNa2S2O3������NaOH��Һ��������_____________________��
�罫��Һ©���е�H2SO4�ij�Ũ���ᣬ��������ƿ�ڳ�Na2S2O3�����⣬����
���ѧʽ���������ɡ�
��2��Ϊ�ⶨ���ñ��շ���Ʒ��Na2S2O3��5H2O���������������ñ�����Һ���еζ���
�÷�Ӧ�Ļ�ѧ����ʽΪ��2Na2S2O3��I2 �� 2NaI��Na2S4O6
������KIO3��KI��HCl��ԭ�Ͽ����Ʊ�����Һ��д������ʱ��������Ӧ�����ӷ���ʽ ��
��ȷ��ȡһ��������Na2S2O3��5H2O��Ʒ����ƿ�У���ˮ�ܽ⣬���μ�______��ָʾ�����������Ƶı�����Һ�ζ����ζ�ʱ���õIJ�����������ƿ�⣬���� ��
�����ζ�ʱ����֣��տ�����Һ�ֲ���ɫ��ֹͣ�ζ������ʹ��Ʒ��Na2S2O3��5H2O�����������IJ������___________���ƫ�ߡ���ƫ�͡����䡱����
��3����ʵ���Na2S�Ĵ���Ҫ��ϸߣ�����ͼ2��ʾ��װ�ÿɽ���ҵ����Na2S�ᴿ����֪Na2S���������ھƾ�������ʱ�ܽ��Ѹ���������ʲ����ھƾ���
�ᴿ��������Ϊ��
�ٽ��ѳ����õĹ�ҵ��Na2S����Բ����ƿ�У�������һ�������ľƾ�������ˮ��
�ڰ�ͼ2��ʾװ����������������������ͨ����ȴˮ��ˮԡ���ȣ�
�۴� ʱ��ֹͣ���ȣ�����ƿȡ�£�
�� __________________________��
�� __________________________��
�����ù���ϴ�ӡ�������ɵõ�Na2S��9H2O���塣
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
��9�֣�ij�о���ѧϰС�����о���������Ư������ʱ���ӡ�������Ư������ʵ������������ˮ��Ӧ���ɵĴ������Ư�����á��õ�������Ϊ��̽�����������Ư�����õ����Ƕ������������Ƕ���������ˮ���õIJ����С�����������ʵ�顣��ش�������⡣
��1��Ϊ��̽��SO2�ܷ�ʹƷ����ɫ����ͬѧѡ������ȷ��ҩƷ�����������ͼ��ʾʵ��װ�ã���ָ��ʵ��װ��ͼ����еIJ�����֮����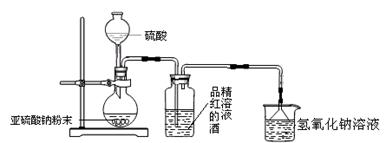
�� ��
�� ��
��2����ͬѧѡ������ȷװ�ú�ʵ���п��ƶ��������Դ�Լÿ��3�����ݵ��ٶ�ͨ��Ʒ��ľƾ���Һʱ������һСʱ��Ʒ���Բ���ɫ��Ϊ�ˣ�����ΪʹƷ���ˮ��Һ��ɫ���������� ��
��3����ͬѧ��һ��ʵ�����£�ȡ������ͬŨ�ȵ�Ʒ��ˮ��Һ����֧�Թ��У��ٷֱ���������������ƹ�������������ƹ��壬��֧�Թ��е�Ʒ�춼��ɫ�����ó����ۣ�ʹƷ����ɫ�����϶���HSO3-��SO32-������Ϊ���Ľ����Ƿ���ȷ �� �������� ��
��4����������װ��̽��SO2��ijЩ��ѧ���ʡ�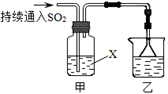
��װ���ҵ������� ��
����XΪNa2S��Һ���۲쵽��Һ�г��ֵ���ɫ���ǣ�˵��SO2���� ��
a�������� b����ԭ��
c��Ư���� d.���ȶ���
�����Լ�XΪCa(ClO)2��Һ���ɹ۲쵽��ɫ�������ɣ���ɸù��̵����ӷ���ʽ�� Ca2��+
Ca2��+ ClO��+
ClO��+ SO2+
SO2+ H2O��
H2O�� ��+
��+ Cl��+
Cl��+ SO42��+
SO42��+ ��
��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
(11��)��ʵ���������Ҵ���ŨH2SO4��Ӧ��ȡ��ϩʱ�������¶ȹ��߶���������Ӧ�������Ҵ���ŨH2SO4��Ӧ����SO2��CO2��ˮ������̿�ڡ�
��1���ñ��Ϊ�١��� ��ʵ��װ�����һ��ʵ�飬����֤������Ӧ��������к�CO2��SO2��ˮ��������װ�õ�����˳���������������ҵ�����
�� �� �� ����
��2��ʵ���װ�â���Aƿ��������________������Ϊ______________��
Bƿ�е�������________��Bƿ��Һ����Ϊ________;
�Ƿ��ܽ�Bƿ��Һ�������Ը������______ (���ǣ���)��
��3��װ�â��мӵĹ���ҩƷ��________װ�â���ʢ����Һ��________��
��4��װ�â�������ϩ�ķ�Ӧ������______����Ҫ��֤�������������ϩ��Ӧ�����������ͨ��ʢ____________��ϴ��ƿ����ͨ��ʢ______���Թ��С� 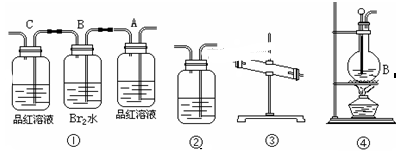
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
�����������壺H2��Cl2��CH4��HCl��NH3��NO��H2S��SO2������ͼװ�ý���ʵ�飬��д���пհף�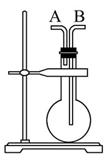
(1)����ƿ����ʱ����A�ڽ������ռ��������ǣߣߣߣߣߣ���B�ڽ������ռ��������ǣߣߣߣߡ�
(2)����ƿ�г���ˮʱ�������������ߣߣߣߵ�����������
(3)����ƿ��װ��ij����Һ������ϴ��ʱ������Ӧ�ӣߣߣߣ߿ڽ�����ƿ��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com