
����Ŀ����A��B��C��D����Ԫ�أ����ǵ�ԭ����������������С��18��A��B��ͬһ���ڣ�A�ĵ���ʽΪ![]() ��Bԭ��L��ĵ���������K���3����0.1 mol C���ʴ������û���2.24 L����(��״��)��ͬʱ�����Neԭ�ӵĵ��Ӳ�ṹ��D���ӵİ뾶��C���ӵ�С��D������B���ӵĵ��Ӳ�ṹ��ͬ��
��Bԭ��L��ĵ���������K���3����0.1 mol C���ʴ������û���2.24 L����(��״��)��ͬʱ�����Neԭ�ӵĵ��Ӳ�ṹ��D���ӵİ뾶��C���ӵ�С��D������B���ӵĵ��Ӳ�ṹ��ͬ��
(1)д��A��B��C��DԪ�ص����ƣ�A______��B______��C______��D______��
(2)DԪ�������ڱ��е�______���ڵ�______�壻
(3)�õ���ʽ��ʾA����̬�⻯����γɹ���_______________________��
(4)A��B�ĵ��ʳ�ַ�Ӧ���ɵĻ�����Ľṹʽ��__________________��
(5)����Ԫ���У�______������������ˮ������������ᣬ�������ڼ����NaOH��Һ�Ļ�ѧ����ʽΪ________________��
���𰸡�̼ �� þ �� �� ��A  O=C=O Al Al(OH)3+NaOH= NaAlO2+2H2O
O=C=O Al Al(OH)3+NaOH= NaAlO2+2H2O
��������
Bԭ��L��ĵ���������K���3��������L����6�����ӣ�����BΪOԪ�أ�A��Bλ��ͬһ���ڣ���A�������4�����ӣ���AΪCԪ�أ�0.1 mol C���ʴ������û���2.24 L����(��״��)����0.1mol������ͬʱ�����Neԭ�ӵĵ��Ӳ�ṹ����C��������Ӧ��������λ����ɣ�����ΪMgԪ�أ�D���ӵİ뾶��C���ӵ�С��D������B���ӵĵ��Ӳ�ṹ��ͬ����Dֻ����λ��Mg֮���ͬ���ڽ���Ԫ�أ���AlԪ�ء�
(1)���ݷ�����֪AΪ̼��BΪ����CΪþ��DΪ����
(2)��ԭ�ӵĺ˵����Ϊ13��λ��Ԫ�����ڱ��ĵ������ڵ���A�壻
(3)A����̬�⻯��ΪCH4�����ڹ��ۻ�����õ���ʽ��ʾ�γɹ��̣�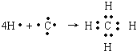 ��
��
(4)̼���ʺ�������ַ�Ӧ���ɶ�����̼�����ڹ��ۻ�����ṹʽΪO=C=O��
(5)��Ԫ�ص�����������ˮ����Al(OH)3�����������ᣬ�������ڼ����NaOH��Һ����ƫ�����ƺ�ˮ������ʽΪAl(OH)3+NaOH= NaAlO2+2H2O��


 ͬ��ѧ��һ�ζ���ϵ�д�
ͬ��ѧ��һ�ζ���ϵ�д� �����ܾ�ϵ�д�
�����ܾ�ϵ�д� ���ƿ�����ϵ�д�
���ƿ�����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����������У����ܷ�����ѧ��Ӧʹ��ˮ��ɫ������ʹ����KMnO4��Һ��ɫ���ǣ� ��
��SO2 ��CH2=CH2 ��![]() ��CH3CH3
��CH3CH3
A.�٢ڢۢ�B.�ۢ�C.�٢ڢ�D.�٢�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����֪�� (1)Fe2O3(s)+![]() C(s)��
C(s)��![]() CO2(g)+2Fe(s)����H��+234.1kJmol-1
CO2(g)+2Fe(s)����H��+234.1kJmol-1
![]() ��
��![]() �Ħ�H�ǣ� ��
�Ħ�H�ǣ� ��
A.��824.4 kJmol-1B.��627.6kJmol-1C.-744.7kJmol-1D.-169.4kJmol-1
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����¯���������з�������Ҫ��ӦΪ��![]() Fe2O3(s) + CO(g)
Fe2O3(s) + CO(g) ![]()
![]() Fe(s) + CO2(g)����֪�÷�Ӧ�ڲ�ͬ�¶��µ�ƽ�ⳣ�����£�
Fe(s) + CO2(g)����֪�÷�Ӧ�ڲ�ͬ�¶��µ�ƽ�ⳣ�����£�
�¶�/�� | 1000 | 1150 | 1300 |
ƽ�ⳣ�� | 4.0 | 3.7 | 3.5 |
��ش��������⣺
��1���÷�Ӧ��ƽ�ⳣ������ʽK=_____________����H________0(����>������<������=��)��
��2����һ���ݻ�Ϊ10L���ܱ������У�1000��ʱ����Fe2O3��CO��1.0 mol����Ӧ����l0 min��ﵽƽ�⡣���ʱ�䷶Χ�ڷ�Ӧ��ƽ����Ӧ������(CO2)=______________________��CO��ƽ��ת����= _____________��
��3������ߣ�2����CO��ƽ��ת���ʣ��ɲ�ȡ�Ĵ�ʩ��_____________��
A������Fe���� B������Fe2O3���� C���Ƴ�����CO2
D����߷�Ӧ�¶� E����С�������ݻ� F��������ʵĴ���
��4����1L���ܱ������У�1300�����������д�ƽ��״̬����_______________��
A | B | C | D | |
n(Fe2O3) | 0.350 | 0.027 | 0.080 | 0.080 |
n(CO) | 0.010 | 0.010 | 0.010 | 0.050 |
n(Fe) | 0.100 | 0.064 | 0.080 | 0.080 |
n(CO2) | 0.035 | 0.088 | 0.040 | 0.050 |
.
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���ڻ���ƽ�����װ��ǿ��ԭ���£�N2H4����ǿ��������H2O2���������ǻ��ʱ��������������N2��ˮ���������ų������ȡ���֪0.4molҺ̬�º�����H2O2��Ӧ�����ɵ�����ˮ�������ų�256.65kJ��������
��1��д���÷�Ӧ���Ȼ�ѧ����ʽ____________________��
��2����֪H2O(l)====H2O(g)����H=+44kJ��mol-1����16 gҺ̬��ȼ�����ɵ�����Һ̬ˮʱ���ų���������________kJ��
��3��������ӦӦ���ڻ���ƽ��������ͷŴ������ȺͿ��ٲ������������⣬����һ����ͻ�����ŵ���________________________��
��4����֪N2(g)+2O2(g)==2 NO2(g)����H=+67.7 kJ��mol-1�� N2H4(g)+O2(g)== N2(g)+2H2O (g)����H= -534 kJ��mol-1�����ݸ�˹����д������NO2��ȫ��Ӧ���ɵ�������̬ˮ���Ȼ�ѧ����ʽ_____________��
��5����֪��N2(g)��2O2(g)��2NO2(g) ��H=+67.7kJ/mol
N2H4(g)��O2(g)��N2(g)��2H2O(g) ��H=-543kJ/mol
1/2H2(g)��1/2F2(g)��HF(g) ��H=-269kJ/mol
H2(g)��1/2O2(g)��H2O(g) ��H=-242kJ/mol
������Ϊ���÷������������������������Ӧ�ͷ����������ºͷ���Ӧ���Ȼ�ѧ����ʽ��
______________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ʯīϩ����ͼa����һ���ɵ���̼ԭ�ӹ��ɵ�ƽ��ṹ����̼���ϣ�ʯīϩ�в���̼ԭ�ӱ���������ƽ��ṹ�ᷢ���ı䣬ת��Ϊ����ʯīϩ����ͼb����
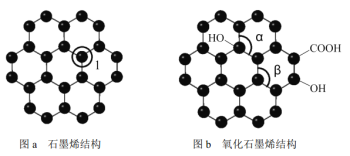
��1��ͼa�У�1��C������C�γɦҼ��ĸ���Ϊ___________,��Cԭ���ӻ���ʽ��______��ͼb�м��Ǧ�_________���Ǧ¡����>�� �� ��<�� ���� ��
��2������ͼ����ʾ������ʯīϩ��ɢ��H2O�У�������ʯīϩ�п���H2O�γ������ԭ����_______����Ԫ�ط��ţ���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ͼ��ʾ��a�ڽ������˳����������֮ǰ��bΪ̼��������˵���в���ȷ����
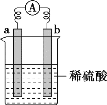
A.a���Ϸ�����ԭ��Ӧ��b���Ϸ���������Ӧ
B.̼�����������ݳ�
C.�������е����������ƶ�����Ϊa��b
D.��Ӧ��a��������С
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�������й�һ�����ʵ���Ũ�ȵ���Һ���ƵĹ����У�������õ���ҺŨ��ƫ�����
A.Ҫ����100 mL 1 mol��L��1 NaOH��Һ���ڰ�ֽ�ϳ�4 g NaOH���壬���ҳ����ٶȽ���
B.����ʱ��������ƿ�̶���
C.�ܽ��ϡ������ʱ�ձ���δ����
D.����ʱ����ƿ�ǣ�ҡ�Ⱥ���Һ����ڿ̶��ߣ��ټ����μ�����ˮʹҺ�����´ﵽ�̶���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ͨ����н�������ʵ�飺
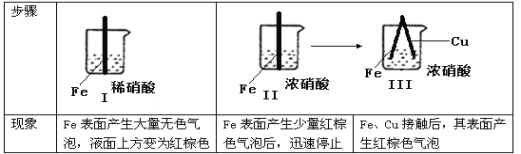
����˵������ȷ���ǣ�
A.���к���ɫ����������ɵĻ�ѧ����ʽΪ��Fe + 6HNO 3== Fe(NO) 3+3H2O + 3NO2
B.���е�����˵��Fe�����γ����ܵ������㣬��ֹFe��һ����Ӧ
C.�Ա�������������˵��ϡHNO3��������ǿ��ŨHNO3
D.�������������Fe��Cu֮�����ӵ����ƣ����ж�Fe�Ƿ�ԭ
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com