
һ���ѧ�о��ɹ�������ͭ��������(CuMn2O4)���ڳ����´����������е�һ����̼�ͼ�ȩ(HCHO)��
(1)��һ�����ʵ���Ũ�ȵ�Cu(NO3)2��Mn(NO3)2��Һ�м���Na2CO3��Һ�����ó������������գ����Ƶ�CuMn2O4��
��Mn2����̬�ĵ����Ų�ʽ�ɱ�ʾΪ ��
��NO3���Ŀռ乹�� (����������)��
(2)��ͭ��������Ĵ��£�CO��������CO2��HCHO��������CO2��H2O��
�ٸ��ݵȵ���ԭ����CO���ӵĽṹʽΪ ��
��H2O������Oԭ�ӹ�����ӻ�����Ϊ ��
��1molCO2�к��еĦҼ���ĿΪ ��
(3)��CuSO4��Һ�м������NaOH��Һ������[Cu(OH)4]2���������ǿռ乹�ͣ�[Cu(OH)4]2���Ľṹ����ʾ��ͼ��ʾΪ ��
 ���б�ˢ��ϵ�д�
���б�ˢ��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��5mol Fe2O3��4mol Fe3O4��3mol FeO��ɵĻ������봿��1mol���ڸ����º�Fe2O3��Ӧ����������ȫ��Ӧ����Ӧ��������FeO��Fe2O3�����ʵ���֮�ȿ�����
A��4:3 B��3:2 C��3:1 D��2:l

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ʯī�ڲ�����������ҪӦ�á�ij����ʯī�к�SiO2(7.8%)��Al2O3(5.1%)��Fe2O3(3.1%)��MgO(0.5%)�����ʡ���Ƶ��ᴿ���ۺ����ù������£�
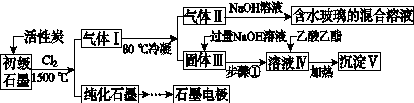
(ע��SiCl4�ķе�Ϊ57.6 �棬�����Ȼ���ķе������150 ��)
(1)��Ӧ����ͨ��Cl2ǰ����ͨһ��ʱ��N2����ҪĿ����____________________��
(2)���·�Ӧ��ʯī�����������ʾ�ת��Ϊ��Ӧ���Ȼ��������е�̼��������ҪΪ________�����������ij��õ�ˮ�����Ļ�ѧ��Ӧ����ʽΪ____________________________________________��
(3)�����Ϊ�����衢________��������Һ���е���������________��
(4)����Һ�����ɳ��������ܷ�Ӧ�����ӷ���ʽΪ______________________________________________��100 kg����ʯī�����ܻ�â�������Ϊ______kg��
(5)ʯī��������Ȼˮ����ͭ���ĵ绯ѧ�����������ͼ����ʾ��ͼ��������Ӧ��ע��
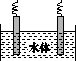
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
FeCl3���ִ���ҵ������Ӧ�ù㷺��ij��ѧ�о���ѧϰС��ģ�ҵ���������Ʊ���ˮFeCl3�����ø���ƷFeCl3��Һ�����ж���H2S��
I.���������ϵ�֪����ˮFeCl3�ڿ������׳��⣬����������������������Ʊ���ˮFeCl3��ʵ�鷽����װ��ʾ��ͼ�����ȼ��г�װ����ȥ���������������£�
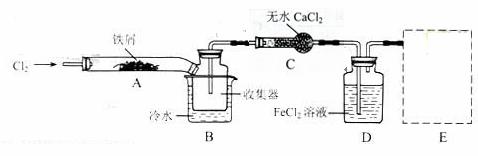
�ټ��װ�õ������ԣ�
��ͨ������Cl2���Ͼ�װ���еĿ�����
���þƾ�������м�·���������Ӧ���
�ܡ���
����ϵ��ȴ��ֹͣͨ��Cl2�����ø����N2�Ͼ�Cl2�����ռ����ܷ�
��ش��������⣺
װ��A�з�Ӧ�Ļ�ѧ����ʽΪ_____________________________________��
�ڢ۲����Ⱥ����ɵ���״FeCl3�ֽ����ռ��������������ڷ�Ӧ��A���Ҷˡ�Ҫʹ������FeCl3�����ռ������ڢܲ�������_______________________________________________��
���������У�Ϊ��ֹFeCl3��������ȡ�Ĵ�ʩ�У������ţ�_________________________��
װ��B�е���ˮԡ������Ϊ__________________��װ��C������Ϊ__________________��װ��D��FeCl2ȫ����Ӧ�����Ϊʧȥ����Cl2�����ö�ʧЧ��д������FeCl2�Ƿ�ʧЧ���Լ���___________��
�����߿��ڻ���β������װ��E��ע���Լ���
II.����ͬѧ��װ��D�еĸ���ƷFeCl3��Һ����H2S���õ��������˺�����ʯīΪ�缫����һ�������µ����Һ��
FeCl3��H2S��Ӧ�����ӷ���ʽΪ___________________________________________________��
������H+�������ŵ����H2�������ĵ缫��ӦΪ___________________________________��
�ۺϷ���ʵ��II��������Ӧ����֪��ʵ�������������ŵ㣺
��H2S��ԭ��������100%����____________________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����þ��ҽҩ����������ҵӦ�ù㷺������þ��ԭ�Ƚ��Ʊ��ߴ�����þ��һ���µ�̽��������þ��(��Ҫ�ɷ�ΪMgCO3��������FeCO3)Ϊԭ���Ʊ��ߴ�����þ��ʵ���������£�
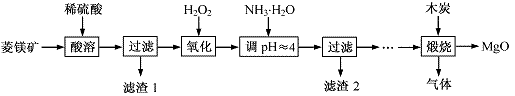
(1)MgCO3��ϡ���ᷴӦ�����ӷ���ʽΪ ��
(2)����H2O2����ʱ��������Ӧ�Ļ�ѧ����ʽΪ ��
(3)����2�ijɷ��� (�ѧʽ)��
 (4)���չ��̴������·�Ӧ��
(4)���չ��̴������·�Ӧ��
2MgSO4��C 2MgO��2SO2����CO2��
2MgO��2SO2����CO2��
MgSO4��C MgO��SO2����CO��
MgO��SO2����CO��
MgSO4��3C MgO��S����3CO��
MgO��S����3CO��
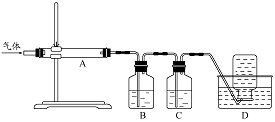
������ͼװ�ö����ղ�����������зֲ����ջ��ռ���
��D���ռ������������ (�ѧʽ)��
��B��ʢ�ŵ���Һ������ (����ĸ)��
a.NaOH ��Һ b.Na2CO3��Һ c.ϡ���� d.KMnO4��Һ
��A�еõ��ĵ���ɫ�������ȵ�NaOH��Һ��Ӧ��������Ԫ�����̬Ϊ��4��д���÷�Ӧ�����ӷ���ʽ�� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�Ȼ�����Ʒ�к�������̼��ء�����غͲ�����ˮ�����ʡ�Ϊ���ᴿ�Ȼ��أ��Ƚ���Ʒ��������ˮ�У���ֽ������ˣ��ڽ���Һ����ͼ��ʾ������в�����
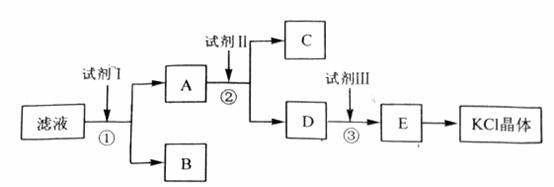
�ش��������⣺
��ʼ��Һ��pH_____________7������ڡ�����С�ڡ����ڡ�������ԭ����_________________________________________________��
�Լ�I�Ļ�ѧʽΪ______________________�����з�����Ӧ�����ӷ���ʽΪ____________________________________________��
�Լ���Ļ�ѧʽΪ______________________�����м����Լ����Ŀ����__________________________________________________________________��
�Լ����������______________________�����з�����Ӧ�����ӷ���ʽΪ__________________________________________________________________��
ijͬѧ��ȡ�ᴿ�IJ�Ʒ0.7759g���ܽ������100mL����ƿ�У�ÿ��ȡ25.00mL��Һ����0.1000mol��L-1������������Һ�ζ������εζ����ı���Һ��ƽ�����Ϊ25.62mL���ò�Ʒ�Ĵ���Ϊ_______________________________________ _____������ʽ����������
_____������ʽ����������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�������������ӳɷ�Ӧ����������( )��
A����ϩʹ����KMnO4��Һ��ɫ B����ϩʹ������Ȼ�̼��Һ��ɫ
C������������ˮ�У���ˮ��ӽ���ɫ D��������������ϣ�����һ��ʱ������ɫ��ʧ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
PHB������һ�ֿ������������½���Ļ��������ϣ���ṹ��ʽΪ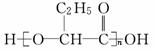 �������й�PHB˵������ȷ���� (�� ��)
�������й�PHB˵������ȷ���� (�� ��)
A��PHBͨ���Ӿ۷�Ӧ�Ƶ�.
B��PHB�ĵ�����CH3CH2CH(OH)COOH
C��PHB�����������µĽ�����������CO2��H2O
D��PHB��һ�־���
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com