
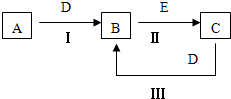
 A��B��C��D��EΪ��ѧ��ѧ�����ĵ��ʻ���ת����ϵ��ͼ��ʾ��
A��B��C��D��EΪ��ѧ��ѧ�����ĵ��ʻ���ת����ϵ��ͼ��ʾ��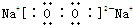
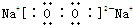
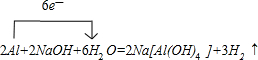
| CO2 |
| E |
| D |
| CO2 |
| NaOH |
| CO2 |
| E |
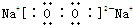 ��
��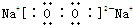 ��
��| D |
| CO2 |
| NaOH |
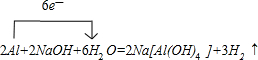 ��
�� ��
��


| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 A��B��C��D��EΪ��ѧ��ѧ�������ʣ�����A��CΪ�������ʣ�EΪ�ǽ������ʣ���ͼ������֮����ת����ϵ����ش�
A��B��C��D��EΪ��ѧ��ѧ�������ʣ�����A��CΪ�������ʣ�EΪ�ǽ������ʣ���ͼ������֮����ת����ϵ����ش�
| ||
| ||
| Cu |
| Cu |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����




�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| AԪ�صĺ���������͵��Ӳ�����ȣ�Ҳ����������ḻ��Ԫ�� |
| BԪ��ԭ�ӵĺ���p��������s��������1 |
| Cԭ�ӵĵ�һ�����ĵ����ֱܷ��ǣ� I1=738kJ/mol I2=1451kJ/mol I3=7733kJ/mol I4=10540kJ/mol |
| Dԭ�Ӻ�������p���ȫ������� |
| EԪ�ص������������������IJ�Ϊ4 |
| F��ǰ�������е縺����С��Ԫ�� |
| G�����ڱ��ĵ����� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com