
��֪25 ��ʱ������ʵĵ���ƽ�ⳣ����
Ka(CH3COOH)��1.8��10��5��Ka(HSCN)��0.13��
(1)��20 mL 0.10 mol��L��1 CH3COOH��Һ��20 mL 0.10 mol��L��1��HSCN��Һ�ֱ���0.10 mol��L��1��NaHCO3��Һ��Ӧ��ʵ���ò���CO2�������(V)��ʱ��t�Ĺ�ϵ��ͼ��ʾ��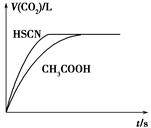
��Ӧ��ʼʱ��������Һ����CO2���������Բ�ͬ��ԭ����________����Ӧ������������Һ��c(SCN��)________c(CH3COO��)(���������������)��
(2)2.0��10��3 mol��L��1�������ˮ��Һ�У�������ҺpH(���Ե���ʱ����仯)�����ƽ����ϵ��c(F��)��c(HF)����ҺpH�Ĺ�ϵ��ͼ��ʾ����25 ��ʱ��HF����ƽ�ⳣ��ΪKa(HF)��________(��ʽ��ֵ)��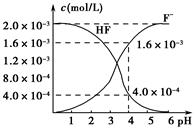
(3)��������CaF2�ܶȻ�����ΪKsp��1.5��10��10����4.0��10��3 mol��L��1 HF��Һ��4.0��10��4 mol��L��1��CaCl2��Һ�������ϣ�������ҺpH��4(���Ե���ʱ��Һ����仯)���Է�����Ϻ��Ƿ��г������ɣ�________(��С���û�С�)���������ɣ�____________________________________________��
 ��˼ά������ҵ���ټ��ִ�ѧ������ϵ�д�
��˼ά������ҵ���ټ��ִ�ѧ������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��0.1 mol/L��������Һ(a)��������Һ(b)��������Һ(c)��50 mL���ԱȽϣ�
(1)�������������Ũ���ɴ�С��˳����________(����ĸ���ţ���ͬ)���������pH�ɴ�С��˳����________��
(2)�������������п��Ӧ����ʼʱ����H2�������ɴ�С��˳����________��(������Zn�Ĵ��ȼ������������)
(3)�������������п��Ӧ����H2������ɴ�С��˳����________��
(4)������ֱ��0.1 mol/L��NaOH��Һ�кͣ�����NaOH��Һ����ɴ�С��˳����________��
(5)������ֱ��50 mL 0.1 mol/L��NaOH��Һ��Ӧ����Һ��pH�ɴ�С��˳����________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��1����25��ʱ����Ũ�Ⱦ�Ϊ0.01 mol��L��1��MgCl2��AlCl3�����Һ����μ��백ˮ��������________����(�ѧʽ)�����ɸó��������ӷ���ʽΪ_____________________��(��֪25��ʱKsp[Mg(OH)2]��1.8��10��11��Ksp[Al(OH)3]��3��10��34��)
��2��ij�¶�(t��)ʱ�����0.01 mol��L��1��NaOH��Һ��pH��11���ڴ��¶��£���pH��2��H2SO4��ҺVaL��pH��12��NaOH��ҺVbL��ϣ������û��ҺΪ���ԣ���Va�UVb��________��
��3����25��ʱ����c mol��L��1�Ĵ�����Һ��0.02 mol��L��1NaOH��Һ�������Ϻ���Һ�պó����ԣ��ú�c�Ĵ���ʽ��ʾCH3COOH�ĵ��볣��Ka��________��
��4��(2013��ɽ���߿�)25��ʱ��H2SO3 HSO3-��H���ĵ��볣��Ka��1��10��2mol��L��1������¶���NaHSO3ˮ�ⷴӦ��ƽ�ⳣ��Kb��________mol��L��1������NaHSO3��Һ�м���������I2������Һ��
HSO3-��H���ĵ��볣��Ka��1��10��2mol��L��1������¶���NaHSO3ˮ�ⷴӦ��ƽ�ⳣ��Kb��________mol��L��1������NaHSO3��Һ�м���������I2������Һ��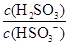 ��________(���������С�����䡱)��
��________(���������С�����䡱)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
����Ũ�Ⱦ�Ϊ0.1 mol��L��1��������Һ��
�����ᡢ�ڴ�����Һ��������������Һ�����Ȼ����Һ���ݴ������Һ�����������Һ�������������Һ���ఱˮ����ش��������⣺
��1���١��ڡ��ۡ���������Һ����ˮ�������H��Ũ���ɴ�С��˳���ǣ�����ţ�________��
��2���ܡ��ݡ��ߡ���������Һ��NH4+Ũ���ɴ�С��˳���ǣ�����ţ�________��
��3�����ۺܵ͢������Ϻ��Һ�и�����Ũ�ȹ�ϵ��ȷ����________��
| A��c��Na������c��Cl������c��OH������c��NH4+�� |
| B��c��Na������0.1 mol��L��1 |
| C��c��Na������c��NH4+����c��Cl������c��OH���� |
| D��c��H������c��OH���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�����£���100 mL 0.01 mol��L��1 HA��Һ����μ���0.02 mol��L��1 MOH��Һ��ͼ����ʾ���߱�ʾ�����Һ��pH�仯���(����仯���Բ���)��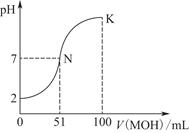
�ش��������⣺
(1)��ͼ����Ϣ��֪HAΪ________��(�ǿ��������)��������________________________________________________��
(2)������һ��Ũ�ȵ�MAϡ��Һ��pH��a����a________________________________________________________7
(�>����<������)�������ӷ���ʽ��ʾ��ԭ��Ϊ_____________________________________________________
��ʱ����Һ����ˮ�������c(OH��)��________��
(3)��д��K������Ӧ����Һ������Ũ�ȵĴ�С��ϵ��_________________________________________��
(4)K���Ӧ����Һ�У�c(M��)��c(MOH)________2c(A��)(�>����<������)������ʱ��Һ�У�pH��10����c(M��)��c(OH��)��________mol��L��1��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
������(H3PO3)�Ƕ�Ԫ�ᣬ������NaOH��Һ��Ӧ����Na2HPO3��
(1)PCl3ˮ�����ȡ�����PCl3��3H2O===H3PO3��________��
(2)H3PO3��Һ�д��ڵ���ƽ�⣺H3PO3 H����H2PO3����
H����H2PO3����
��ij�¶��£�0.10 mol��L��1��H3PO3��ҺpH��1.6������Һ��c(H��)��2.5��
10��2 mol��L��1������¶�����������ƽ���ƽ�ⳣ��K��д��������̡�(H3PO3�ĵڶ���������Բ��ƣ����������λ��Ч����)
�ڸ���H3PO3�����ʿ��Ʋ�Na2HPO3ϡ��Һ��pH________7(�>����������<��)��
(3)���������ǿ��ԭ�ԣ���ʹ��ˮ��ɫ���÷�Ӧ�Ļ�ѧ����ʽΪ________��
(4)���Na2HPO3��ҺҲ�ɵõ������ᣬװ��ʾ��ͼ���£�
�������ĵ缫��ӦʽΪ________________________��
�ڲ�Ʒ���з�Ӧ�����ӷ���ʽΪ_____________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�����£���0.05 mol��L��1������Һ��δ֪Ũ�ȵ�NaOH��Һ��1��2������Ȼ�ϣ�������Һ��pH��12��������NaOH��Һ12.5 mL��pH��3��ijһԪ����HA��Һ20.0mLǡ����ȫ��Ӧ����NaA��
��1����һԪ��������ʵ���Ũ��Ϊ ��
��2���������¸�һԪ����ĵ���ƽ�ⳣ��Ϊ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
����A�����ᣬB�����ᣬ C�����������ᣬ�������������ź͡�������������������ա�
��1����ͬ�����ͬpH���������У��ֱ����������NaHCO3��ĩ������ͬ�����²���CO2������ɴ�С��˳���� ��
��2����ͬ�����ͬ���ʵ���Ũ�ȵ��������У��ֱ����������NaHCO3��ĩ������ͬ�����²���CO2������ɴ�С��˳���� ��
��3�����ʵ���Ũ�Ⱦ�Ϊ0��1mol/L����������Һ��pH�ɴ�С��˳���� �����ȡ�������0��1mol/L����������Һ����0��1mol/L��NaOH�кͣ���ǡ����ȫ��Ӧʱ������NaOH��Һ������ɴ�С��˳���� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
̽��С���õζ����ⶨCuSO4��5H2O(Mr��250)������ȡa g�������100 mL��Һ��ÿ��ȡ20.00 mL�������������Ӻ���c mol ��L��1 EDTA(H2Y2��)����Һ�ζ����յ㣬ƽ������EDTA��Һb mL���ζ���Ӧ���£�
Cu2����H2Y2��=CuY2����2H��
(1)д������CuSO4��5H2O���������ı���ʽw��_______________��
(2)���в����ᵼ��CuSO4��5H2O�����IJⶨ���ƫ�ߵ���________��
a��δ������ƿ
b���ζ��յ�ʱ�ζ��ܼ����в�������
c��δ��������EDTA��Ӧ�ĸ�������
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com