
��֪���صĽṹʽΪ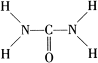 �� ���ؿ��������л����ʣ���Ҫ�������������������غ���������ѧʽΪ��Fe��H2NCONH2��6�ݣ�NO3��3��
�� ���ؿ��������л����ʣ���Ҫ�������������������غ���������ѧʽΪ��Fe��H2NCONH2��6�ݣ�NO3��3��
��1����̬Fe3+�ĺ�������Ų�ʽΪ ��C��N��O����Ԫ�صĵ�һ�������ɴ�С��˳���� ��
��2�����ط�����Nԭ�ӵ��ӻ���ʽ�� ��
��3��NH+4��H��N��H���DZ�NH3��H��N��H���Ǵ�ԭ��Ϊ ��
��4��CO2��NH3�ǹ�ҵ���Ʊ����ص���Ҫԭ�ϣ���̬CO2���ɱ����ľ����ṹ����ͼ��ʾ��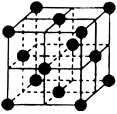
��1��CO2������Χ�Ⱦ����Ҿ��������CO2������ ����
��NaCl����ҲΪ���������ṹ����֪NaCl�����ܶ�Ϊ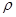 g��cm-3��NA��ʾ�����ӵ���������NaCl�������Ϊ cm3
g��cm-3��NA��ʾ�����ӵ���������NaCl�������Ϊ cm3
��8�ݣ���1��1s22s22p63s23p63d5����[Ar]3d5����2�֣� N��O��C��2�֣� ��3��sp3��1�֣�
��4��NH3��Nԭ������1�Թ¶Ե��ӣ�NH+4��Nԭ����û�й¶Ե��ӣ��ų�����С��1�֣�
��4����12��1�֣� ��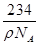 ��1�֣�
��1�֣�
���������������1��Feԭ�Ӻ�����26�����ӣ���������Ų�Ϊ1s22s22p63s23p63d64s2��Feԭ��ʧȥ4s�ܼ�2�����ӡ�3d�ܼ�1�������γ�Fe3+��Fe3+�����Ų�ʽΪ1s22s22p63s23p63d5��ͬ����������ҵ�һ�����ܳ��������ƣ�NԪ��ԭ�ӵ�2p�ܼ���3�����ӣ�Ϊ�����ȶ�״̬���������ͣ�ʧȥ������Ҫ�������ϸߣ���һ�����ܸ���ͬ��������Ԫ�أ��ʵ�һ������N��O��C��
��2�������صĽṹʽ��֪�����ط�����Nԭ�ӳ�3������������1�Թ¶Ե��ӣ��ӻ������Ϊ4�����Nԭ�Ӳ�ȡsp3�ӻ���
��3��NH3��Nԭ���Ϻ���1�Թ¶Ե��ӣ�NH+4��Nԭ����û�й¶Ե��ӣ����NH+4��N��H��֮����ų�����С����NH+4��H��N��H���Ǵ�
��4�����Զ���Ķ�����̼����Ϊ���ģ���֮����Ķ�����̼����λ�������ϡ���ÿ�������к���3��CO2���ӡ�����ÿ����������γ�8����ͬ�������壬����ÿ���汻2�������干�ã�������֮����Ķ�����̼�����У�8��3����2��12��
�������㡢����Ϊ�����ӣ������ġ�������Ϊ�����ӣ����к��е���������ĿΪ8��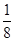 ��6��
��6��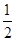 ��4������������Ŀ��1��12��
��4������������Ŀ��1��12��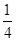 ��4�����ʾ������൱�ں���4����NaCl�����ӣ���������Ϊ
��4�����ʾ������൱�ں���4����NaCl�����ӣ���������Ϊ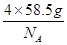 ��NaCl�����ܶ�Ϊ��g?cm-3���ʾ��������Ϊ
��NaCl�����ܶ�Ϊ��g?cm-3���ʾ��������Ϊ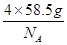 �¦�g?cm-3��
�¦�g?cm-3��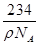 cm3��
cm3��
���㣺�����������Ų����ɡ��ӻ�������ۡ���һ�����ܱȽϡ��۲���ӶԻ������ۡ������ṹ�뾧������ļ����



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
����ԭ�ӻ��ȡ������ܡ����ܵĵ�λ����kJ��mol-1
| ���� | ����ԭ�ӻ��� | ���ӻ����� | ������ | ���ۼ� | ���� |
| Na | 108.4 | NaCl | 786 | Cl-Cl | 243 |
| Mg | 146.4 | NaBr | 747 | Si-Si | 176 |
| Al | 326.4 | MgO | 3791 | Si-Cl | 360 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
(9��)��֪A��B��C��D��E����Ԫ�����ڱ���ǰ36�ŵ�Ԫ�أ����ǵ�ԭ��������������A ������4��Ԫ�ؼȲ���ͬһ�����ֲ���ͬһ���塣B��C��ͬһ���壬D��E��ͬһ���ڣ���֪E�����ڱ���1��18���еĵ�7��Ԫ�ء�D��ԭ��������EС5��D��B���γ����ӻ������侧���ṹ����ͼ�� 
 ��1��A��Ԫ�ط����� ��E��Ԫ�����ڱ��е�λ���� ������+2�����ӵĵ����Ų�ʽΪ ��
��1��A��Ԫ�ط����� ��E��Ԫ�����ڱ��е�λ���� ������+2�����ӵĵ����Ų�ʽΪ ��  ��2��B���⻯��ľ��������� ���壬B���⻯����C���⻯����ȣ����Ӽ��Խϴ���� ��д��ѧʽ����
��2��B���⻯��ľ��������� ���壬B���⻯����C���⻯����ȣ����Ӽ��Խϴ���� ��д��ѧʽ���� ��3����ͼ�п��Կ�����D��B�γɵ����ӻ�����ĵ���ʽΪ �������ӻ����ᄃ����ܶ�Ϊag��cm-3����������� ��ֻҪ���г���ʽ����
��3����ͼ�п��Կ�����D��B�γɵ����ӻ�����ĵ���ʽΪ �������ӻ����ᄃ����ܶ�Ϊag��cm-3����������� ��ֻҪ���г���ʽ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
���мס��ҡ������־���ľ���������X���ھ��������ģ�����A���ھ��������ģ�������֪��������X��Y�ĸ�������________������A��B�ĸ�������________������������________��C���ӣ���________��D���ӡ�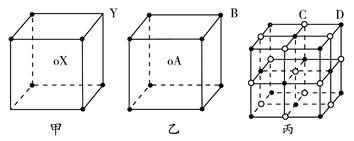
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
A��B��D��E��G��M�������ֳ���Ԫ�أ����ǵĺ˵���������������У�Ԫ��M�Ļ�̬3d�������2�����ӣ�A�Ļ�̬ԭ��L���������K���������2����E�ļ�������ͬ����Ԫ�صļ������а뾶��С��D��Gͬ���壻B��D�γɵĻ������ж��֣�����һ���Ǻ���ɫ���塣
��1��M�Ļ�̬ԭ�Ӽ۲�����Ų�ʽΪ_____,Ԫ��B��D��G�ĵ�һ�������ɴ�ʱС��˳����_____ (��Ԫ�ط��ű�ʾ)��
��2���ü۲���ӶԻ�������Ԥ�⣬GD32�������幹����_____ (�����ֱ���)
��3��M��D�γɵ�һ�ֳȺ�ɫ���徧���ṹ��ͼ��ʾ���仯ѧʽΪ_____ (��Ԫ�ط��ű�ʾ)��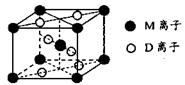
��4����֪������EB�ṹ�뵥�������ƣ������ʿ���E ���Ȼ�����NaB3�ڸ����·�Ӧ�Ƶã������ɵ���B2���÷�Ӧ��ѧ����ʽΪ_____������8.4gB2���ɣ���ת�Ƶ�����Ϊ_____��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��ʴ���
��B��������Al�����أ�Ga������������Ԫ�أ��ڢ�A�壩�����ǵĻ��������
������Ҫ��;���ش��������⣺
��1��д����̬��ԭ�ӵĵ����Ų�ʽ ��
��2����֪����ˮ�Ȼ�����178�����������������ǵϵ�˫���ڣ�Al2Cl6������
���¶���Al2Cl6���������A1Cl3�����ӡ�
�ٹ����Ȼ����ľ��������� ��
��д��Al2Cl6���ӵĽṹʽ ��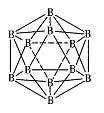 �۵�����A1Cl3�����幹���� ����˫����Al2Cl6��Alԭ�ӵĹ���ӻ������� ��
�۵�����A1Cl3�����幹���� ����˫����Al2Cl6��Alԭ�ӵĹ���ӻ������� ��
��3��������Ľṹ��Ԫ������ʮ���壬ÿ����Ԫ����12����ԭ�裨��ͼ��
����������ԭ��Ϊ10 B������Ϊ11B����ýṹ��Ԫ�� �ֲ�ͬ�Ľṹ
���͡�
��4����������������ϵ���侧���߳�Ϊ405 pm���ܶ���2��70g��cm��3������ȷ
���侧�������ͣ������Ļ����������� �������о����������ԭ
�ӿɿ����ǽӴ��ģ���ʽ��������ԭ�Ӱ뾶r(A1)= pm��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
���ڷ��ȷ�ӦH2 + Cl2  2HCl������˵����ȷ����
2HCl������˵����ȷ����
| A���÷�Ӧ�漰�������Ӽ����ۼ��Ķ������γ� |
| B����Ӧ�������е����������ڲ��������е������� |
| C���Ͽ�1 mol H��H����1 mol Cl��Cl���������յ���������С���γ�1 mol H��Cl�������ų������� |
| D���÷�Ӧ�У���ѧ��ֻת��Ϊ���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
�ҹ���ѧ�ҽ�ʯīϩ(��ʯī����IJ�״�ṹ)��̼���ܾ�������ˮ�����������һ�֡�������ϡ����ò��Ͼ��г�ǿ��������������������Ϊ����Ĵ��ܱ��¡���������������ϡ������й�˵����ȷ����(����)
| A��ʯīϩ�����л��� | B���ò���������Ϊ�����仯 |
| C��̼������̼��һ��ͬλ�� | D��̼�������ڽ����ɢϵ |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com