
( 14��)
(1)���������ڵ�����Ϊ���ʣ�����ȥ���и�������������������Լ���д�ں����ϣ�
��(�ױ�) ����(�Ҵ�) ���ױ�(��) ��
��2��ʵ�����ɵ�ʯ�е�̼���ƺ�ˮ��Ӧ��ȡ��Ȳ���÷�Ӧ�Ļ�ѧ����ʽ Ϊ ��ʵ����Ϊ�˼�����Ӧ���ʣ����� ����ˮ����ʵ���в���������������ŵ���ζ��������_________________�����Լ������Գ�ȥ��
Ϊ ��ʵ����Ϊ�˼�����Ӧ���ʣ����� ����ˮ����ʵ���в���������������ŵ���ζ��������_________________�����Լ������Գ�ȥ��
��3��ij��ȤС���ͬѧ��ʵ��������ȡ����ϩ�г������������� �����������������ʵ��ͼ��ȷ�����������������C2H4��SO2���ش��������⣺
�����������������ʵ��ͼ��ȷ�����������������C2H4��SO2���ش��������⣺
1��I��II��III��IVװ�ÿ�ʢ�ŵ��Լ�����Ϊ  ������ĸ��
������ĸ��
��Ʒ����Һ ��NaOH��Һ ��Ũ���� ������KMnO4��Һ
| A���ܢڢ٢� | B���٢ڢ٢� | C���٢ڢ٢� | D���ܢڢ٢� |
 ��˼ά������ҵϵ�д�
��˼ά������ҵϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
(14��)
(1)��ͼ��A��K�ֱ�����йط�Ӧ��һ�ַ�Ӧ������������A��C��F��K�ǹ��塣��֪A��һ�ֲ�������Ԫ�ص��Σ����ȷֽ��ܵõ����ʵ�����ȵ����ֲ��A���Ⱥ����ɵĻ��������ͨ����ʯ�ң�ֻʣ������B(BΪ��ɫ�д̼�����ζ������)����ͨ��Ũ������ֻʣ������D(DΪ��ɫ��ζ����)��CΪ����ɫ���塣�����ʼ��ת����ϵ����ͼ��ʾ��
��ش��������⣺
��д��C�ĵ���ʽ�� ��
��д��A����Һ������NaOH��Һ��Ӧ�����ӷ���ʽ��
��
��д��ʵ������ȡB�Ļ�ѧ����ʽ��
��
��д��J��K��Ӧ�Ļ�ѧ���̣�
��
(2)����̽��ڻ��Ǵ����м�������M��������ʾ��M��������ԭ�ӷ��ӣ�����Է�������Ϊ60���ڵ�����M�ֽ⡣��ĩ״��KSCN��95%��������һ�������·�Ӧ�ɵõ�����M�������������Σ�����������ʵ���֮����1�s1�s1���������������εĻ�ѧʽΪ �� ������M�Ľṹʽ�� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010��������е�����ѧ�����ڶ���ģ�⿼�� ���ͣ������
(14��)�����£���ijһԪ��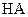 ��
��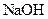 ��Һ�������ϣ����ǰ������Һ��Ũ�Ⱥͻ�Ϻ�������Һ��
��Һ�������ϣ����ǰ������Һ��Ũ�Ⱥͻ�Ϻ�������Һ�� ���±�(���Ϻ���Һ����仯���Բ���)��
���±�(���Ϻ���Һ����仯���Բ���)��
| ʵ���� | 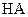 ���ʵ���Ũ�� ���ʵ���Ũ���� 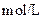 �� �� | 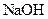 ���ʵ���Ũ�� ���ʵ���Ũ���� 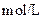 �� �� | �����Һ�� |
| �� | 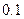 | 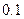 |  |
| �� |  |  |  |
| �� |  | 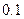 |  |
| �� |  |  |  |
 ��ȡֵ��ΧΪ ��
��ȡֵ��ΧΪ �� �Ƿ�һ������
�Ƿ�һ������ (ѡ��ǡ���)����
(ѡ��ǡ���)����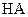 Ϊ���ᣬ������û����Һ��ƽ��ʱ
Ϊ���ᣬ������û����Һ��ƽ��ʱ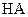 ���볣��
���볣�� �Ľ���ֵ�� (�ú�
�Ľ���ֵ�� (�ú� ����ʽ��ʾ)��
����ʽ��ʾ)�� ��ԭ���� ����������Һ������Ũ�ȵĴ�С��ϵ�� ��
��ԭ���� ����������Һ������Ũ�ȵĴ�С��ϵ�� ��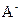 �ڸ������µ�ˮ����� ������Һ�У�ˮ�������
�ڸ������µ�ˮ����� ������Һ�У�ˮ������� ��
��  ��
���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014������ʡ�ɽ�ظ�һ��ѧ����ĩ������⻯ѧ�Ծ��������棩 ���ͣ��ƶ���
(14�֣��±���Ԫ�����ڱ���ǰ�����ڣ��ش��������⣺

��1�������ڰ뵼����ϵ�Ԫ���� (��Ԫ�ط��ţ�������Ԫ�����ڱ��е�λ��Ϊ ��
��2��A��G����Ԫ�طֱ���EԪ�ض����γ����ֻ���������������ӻ�������� ��д��ѧʽ����ͬ�������ڹ��ۻ�������� �������ֻ������к��зǼ��Լ��Ļ������� ��
��3��������ʮ��Ԫ�ص�����������Ӧ��ˮ�����У�������ǿ���� ��������������������� ���û�ѧʽ��ʾ����
��4��ֻ����A��C����Ԫ�صĻ�������� ����Щ����������Է���������С���� ���û�������ӵĿռ乹���� ��
��5������Ԫ��H��ԭ�ӽṹʾ��ͼ ��Ԫ��H��Ԫ��J��ɵĻ������ˮ��Һ�м��������ռ���Һ����Ӧ�����ӷ���ʽΪ ��
��6����Ԫ��A��D��J��ɵļȺ������Ӽ��ֺ��й��ۼ��Ļ�����Ļ�ѧʽΪ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013�����ɹŸ߶���ѧ����ĩ���Ի�ѧ�Ծ��������棩 ���ͣ������
(14��)(1)�ڷ�Ӧ2KMnO4��16HBr===5Br2��2MnBr2��2KBr��8H2O�У���ԭ���� ��
(2)��֪BrFx��H2O�����ʵ���֮��3��5��Ӧ�IJ�����HF��HBrO3��Br2��O2���÷�Ӧ�е��������� ����ԭ���� _��BrFx�е�x��____________��
(3)Ũ�����ڷ�ӦKClO3��HCl�D��KCl��ClO2��Cl2�� (��������)����ʾ������������
_��
(4)��һ�������£�PbO2��Cr3����Ӧ��������Cr2O��Pb2��������1 mol Cr3����Ӧ����PbO2�����ʵ���Ϊ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013������и߶���ѧ����ĩ���Ի�ѧ�Ծ� ���ͣ������
(14��) �����£���ijһԪ��HA��NaOH��Һ�������ϣ�������Һ��Ũ�Ⱥͻ�Ϻ�������Һ��pH���±���
|
ʵ���� |
HA���ʵ���Ũ�ȣ�mol/L�� |
NaOH���ʵ���Ũ�ȣ�mol/L�� |
��Ϻ���Һ��pH |
|
�� |
0.2 |
0.2 |
pH = a |
|
�� |
C1 |
0.2 |
pH = 7 |
|
�� |
0.2 |
0.1 |
pH >8 |
|
�� |
0.1 |
0.1 |
pH = 9 |
��ش�
��1���������������ʵ���������Ӽ�����������������a��˵��HA��ǿ�ỹ�����ᡣ
_______________________________________________________________________________��
��2���������������ʵ�����������������������C1�Ƿ�һ������0.2 mol/L________ �� ��Ϻ���Һ������Ũ��c(A-)��c(Na+)�Ĵ�С��ϵ��c(A-)_______ c(Na+) (��>��<��=)��
(3)�ӱ���ʵ����������HA��_______��(ǿ����)���û��Һ�е�����Ũ���ɴ�С��˳����______________________________________
��4��������Һ�У���ˮ�����c(H��) = mol��L-1��c (Na+)��c (A-)= mol��L-1��
��5����֪NH4A��ҺΪ���ԣ���֪��HA��Һ�ӵ�Na2CO3��Һ��������ų������ƶϣ�NH4��2CO3��Һ��pH 7 (��>��<��= )��
��6������ͬ�¶�����ͬŨ�ȵ���������Һ��
A��NH4HCO3 B��NH4A C��(NH4)2SO4 D��NH4Cl
��c(NH4+)�ɴ�С��˳������ ������ţ�
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com