

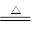 CO2����4NO2����2H2O
CO2����4NO2����2H2O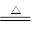 CO2����4NO2����2H2O��
CO2����4NO2����2H2O��

 ͬ����ϰǿ����չϵ�д�
ͬ����ϰǿ����չϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A����ѧ��Ӧ�����ء������غ㡱���ɣ�Ҳ���ء������غ㡱���� |
| B��HF��HCl��HBr��HI�ĵ��ȶ���������ǿ |
| C������ԭ���γɵ����ӣ�һ������ϡ������Ԫ��ԭ�ӵĺ�������Ų� |
| D���������ӣ������������Ų���ͬ�������ǻ�Ϊͬλ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
 Y ��a+c
Y ��a+c Z ��X+Y
Z ��X+Y m ��X+Z
m ��X+Z c+n ��Y+Z
c+n ��Y+Z c+n
c+n�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������

| 25��ƽ����ϵ������ˮ��HA�� | ƽ�ⳣ�� | �ʱ� | ��ʼ��Ũ�� |
��ˮ�У�HA H����A�� H����A�� | K1 | ��H1 | 3.0��10��3 mol��L��1 |
�ڱ��У�2HA (HA)2 (HA)2 | K2 | ��H2 | 4.0��10��3 mol��L��1 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
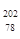 Pt��
Pt��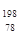 Pt��˵����ȷ����
Pt��˵����ȷ����A��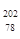 Pt�� Pt��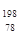 Pt��ͬһ�ֺ��� Pt��ͬһ�ֺ��� | B��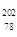 Pt�� Pt��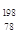 Pt����������ͬ Pt����������ͬ |
C��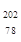 Pt�� Pt��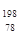 Pt��Ϊͬλ�� Pt��Ϊͬλ�� | D��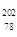 Pt�� Pt��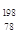 Pt����������Ϊ78 Pt����������Ϊ78 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
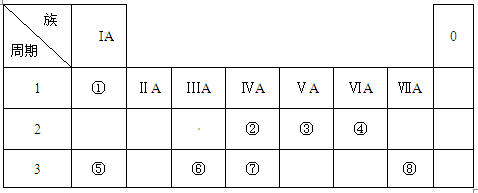
 ��2���ڡ��ۡ��ߵ���ۺ������������ǿ������˳����____________��
��2���ڡ��ۡ��ߵ���ۺ������������ǿ������˳����____________�� ��3���١��ܡ��ݡ����е�ijЩԪ�ؿ��γɼȺ����Ӽ��ֺ����Թ��ۼ��Ļ����д������һ�ֻ�����ĵ���ʽ��____________________��
��3���١��ܡ��ݡ����е�ijЩԪ�ؿ��γɼȺ����Ӽ��ֺ����Թ��ۼ��Ļ����д������һ�ֻ�����ĵ���ʽ��____________________�� ��4���ɱ�������Ԫ�ص�ԭ�Ӱ�1��1��ɵij���Һ̬�������ϡҺ�ױ����ֽ⣬��ʹ�õĴ���Ϊ������ţ�_________________��
��4���ɱ�������Ԫ�ص�ԭ�Ӱ�1��1��ɵij���Һ̬�������ϡҺ�ױ����ֽ⣬��ʹ�õĴ���Ϊ������ţ�_________________�� a.MnO2 b.FeCl3 c.Na2SO3 d.KMnO4
a.MnO2 b.FeCl3 c.Na2SO3 d.KMnO4 ��5���ɱ���Ԫ���γɵij�������X��Y��Z��M��N�ɷ������·�Ӧ��
��5���ɱ���Ԫ���γɵij�������X��Y��Z��M��N�ɷ������·�Ӧ��
 X��Һ��Y��Һ��Ӧ�����ӷ���ʽΪ_____________________��
X��Һ��Y��Һ��Ӧ�����ӷ���ʽΪ_____________________�� N���ĵ��ʵĻ�ѧ����ʽΪ________________��
N���ĵ��ʵĻ�ѧ����ʽΪ________________���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��Na3N�����ᷴӦʱ����һ���� |
| B��Na3N��������Na+�İ뾶��N3-�İ뾶С |
| C����Na3N��ˮ��Ӧ��Na3N�ǻ�ԭ�� |
| D��Na+�� N3-�ĵ��Ӳ㶼���ԭ�ӵĽṹ��ͬ |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com