
��ҵ̼����(����ԼΪ98��)�к���Mg2����Fe2����Cl����SO42�������ʣ��ᴿ�����������£�
 |
��֪��̼���Ƶı�����Һ�ڲ�ͬ�¶�����������������ͼ��ʾ��
 Na2CO3��10H2O Na2CO3��7H2O Na2CO3��H2O
Na2CO3��10H2O Na2CO3��7H2O Na2CO3��H2O
32 36 t���棩
�ش��������⣺
��1���ܽ�ʱͨ���ȿ����������� (д����������)������������þ���������ӷ���ʽΪ ��
��2�������ȹ��ˡ�ʱ���¶�Ӧ������ ���������˺�ϴ�ӹ���IJ��� ��
��3��Ϊ����ĸҺ���Ƿ���Cl-�����������Լ�Ϊ ��
��4��ʵ���ҽ��С����ա����մ������� ��
 ��5����������ʯī�缫��ⱥ��Na2CO3��Һ��װ����ͼ����д��Y�缫
��5����������ʯī�缫��ⱥ��Na2CO3��Һ��װ����ͼ����д��Y�缫
��Ӧʽ�� ��һ��ʱ���X�缫���ռ�����
����һ���� �������� ��
 |
��1�����¼ӿ��ܽ����ʡ���ȥ��Ԫ�ء�����ȴٽ�Fe3+��Fe2+����ˮ�������Fe2+����2�֣���Mg2+ +2OH��= Mg (OH) 2������MgCO3 +2OH��= Mg (OH) 2+CO32���� ��2�֣�
��2��������36�棨2�֣���
��������м���36�����ˮû��������棬��ˮ��Ȼ���ɺ��ظ�����2~3�Σ�2�֣�
��3��������Ba(NO3)2��HNO3��Һ��1�֣���������AgNO3��Һ��1�֣�
��4�������������ǣ�2�֣�����ƾ��Ʋ����۷֣�������һ������1�֣�ֱ����С��0�֣�
��5��2H+ +2e- =H2������2H2O +2e- = H2��+2OH-��2�֣���
O2��CO2��д����Ҳ���֣���1�֣���2�֣�


 �����Ƹ���ʦ����ϵ�д�
�����Ƹ���ʦ����ϵ�д� ��ͨ����ͬ����ϰ��ϵ�д�
��ͨ����ͬ����ϰ��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���շ�ӦBr+H2��HBr+H�������淴Ӧ���̱仯��ʾ��ͼ��������������ȷ����
A����Ӧ����е�������������������е�������
B������ӦΪ���ȷ�Ӧ
C���÷�Ӧ���淴Ӧ�����ȹ���
D��ͼ�п��Կ�����HBr������һ������H2������

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��pH��1����Һ�У��ܴ��������һ�����ӻ������ (����)��
A��Mg2����Na����ClO����NO3��
B��Al3����NH4����Br����Cl��
C��K����Cr2O72����CH3CHO��SO42��
D��Na����K����SiO32����Cl��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�Ͼ�Ӳ�ʺϽ��к�̼����(WC)��������(Co)������������������ ��ⷨ�ɻ���WC��Co���������̼�ͼ���£�
��ⷨ�ɻ���WC��Co���������̼�ͼ���£�

 (1)���ʱ�Ͼɵ������������������������HCl��ҺΪ���Һ��������Ҫ�ĵ缫��ӦʽΪ____________________________________________________��
(1)���ʱ�Ͼɵ������������������������HCl��ҺΪ���Һ��������Ҫ�ĵ缫��ӦʽΪ____________________________________________________��
(2)�������������˱�����Ҫ�ɷ���____________�����յ�ϴ��Һ���� ˮ���Ƶ��Һ��Ŀ���ǻ����������е�____________��
ˮ���Ƶ��Һ��Ŀ���ǻ����������е�____________��
(3)��Һ�����Ҫ�ɷ���____________��ϴ��CoC2O4����ֶ����ղ�Ʒ���Ȳ�������Ӱ�죬������ʱ����ɻ�����Ⱦ��ԭ����_______________________ _________________________________________________��
(4)��Co2O3��ԭ��Co�۵Ļ�ѧ��Ӧ����ʽΪ________________________ _________________________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��������I������II����ȷ���������ϵ����
| ѡ�� | ����I | ����II |
| A | H2�л�ԭ�ԣ�Ũ������ǿ������ | ������Ũ�������H2 |
| B | CuS������ˮ������ | ��Ӧ��H2S+CuSO4=CuS��+H2SO4���Է��� |
| C | ��ˮ�к��д�����Cl-��Br-������ | ��ˮ��ͨ��F2�ɴ������Cl2��Br2 |
| D | SO2�������Ժ�Ư���� | ����ɫʯ����Һ��ͨ��SO2����Һ�ȱ������ɫ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����������ӷ���ʽ��д��ȷ���� (����) ��
��
A��NaClO��Һ��FeCl2��Һ��ϣ�6Fe2����3ClO����3H2O===2Fe(OH)3����
3Cl����4Fe3��
B����ʳ���������е�̼��ƣ�CaCO3��2H��===Ca2����CO2����H2O
C��FeCl2������Һ���ڿ����б��ʣ�2Fe2����4H����O2===2Fe3����2H2O
D�����MgCl2ˮ��Һ�����ӷ���ʽ��2Cl����2H2O H2����Cl2����2OH��
H2����Cl2����2OH��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ij�����ų�����ˮ�к��д�����Fe2����Zn2����Hg2�����ֽ������ӡ�������ij��ѧ�о���ѧϰС���ͬѧ��Ƴ�ȥ��ˮ�еĽ������ӣ��������̷���𩷯(ZnSO4��7H2O)���ķ�����
��ҩƷ����NaOH��Һ��������Һ����������ϡ���ᡢ����
��ʵ�鷽����
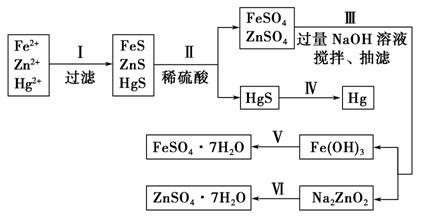
������̽����
(1)�������������Ӧ�����ӷ���ʽΪ_________________________________��
(2)������г��˵�Ŀ����____________���ò������Fe(OH)3�ķ�Ӧ�����ӷ���ʽΪ_________________ _____________________________________��
_____________________________________��
(3)������еõ�����п��Һ�����ӷ���ʽΪ___________________________ _____________________________________________________________��
(4)��ʵ�ֲ�������������Լ���________��________�����漰����Ҫ��������Ϊ__________________________________________________________��
(5)��������õķ�����________���ò����Ƿ�Ի�����Ӱ�죿________(��ǡ��� ��)������Ӱ�죬�������һ����ɫ����������ʵ�ֲ�����ķ�Ӧ��_____________________________________________________________��
��)������Ӱ�죬�������һ����ɫ����������ʵ�ֲ�����ķ�Ӧ��_____________________________________________________________��
(6)���о�С���ͬѧ��ǿ����Һ�У��ô���������Fe(OH)3��Ӧ����˸�Ч��ˮ��Na2FeO4���÷�Ӧ�����ӷ���ʽΪ______________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��������˳����ȷ���ǣ� ��
A�����ȶ��ԣ�CH4>SiH4>HF B.ԭ�Ӱ뾶��Na>Mg>O
C�����ԣ�HClO4>H2SO4>H3PO4 D.�ǽ����ԣ�F>Cl>Br
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�����й����ʱ仯�ͷ����˵����ȷ���� (����)��
A���������̬��Al2O3��12Cת��Ϊ14C�����ڻ�ѧ�仯
B����������ˮ�������������������ǻ����
C����������Һ�͵�����Һ�ı����������ܷ���������ЧӦD��SiO2��NO2��Al2O3����������������
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com