
��Ȼ��������Ҫ����Դ��Ҳ����Ҫ�Ļ���ԭ�ϣ�����Ҫ�ɷ��Ǽ��顣
��1��������һ�������¿����ɣ�
A��̼�����ӣ�CH3-������������ B��̼�����ӣ�CH3-��
C��������CH3�� D��̼ϩ��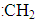 ��
��
���������У�����Ϊ120�����________��������ţ�
����̼�����ӣ�CH3-����Ϊ�ȵ������һ�ַ��ӵĽṹʽΪ_________________________________________________________��
��2�������ش����Ŀ�ȼ���������ˮ��������ڿ�ȼ����˵������ȷ����________��
A�����������ˮ���Ӿ��Ǽ��Է���
B����ȼ���м��������ˮ���Ӽ����������
C����ȼ������ԭ�Ӿ���
D�����������ˮ�����еĦҼ�����s��sp3�������ص����ɵ�
��3���ڸ����£�����ɻ�ԭCuO�õ�Cu2O��
��Cu���ĺ�������Ų�ʽΪ____________��
��Cu2O����ľ����ṹ��ͼ��ʾ�����С��������������ӷ���Ϊ________��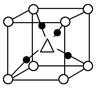
��4��һ�������£�������ˮ����������H2��CO���������ɵ������ЦҼ�������м�����֮��Ϊ________��

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
NaF��NaI����MgO��Ϊ���Ӿ��壬�й��������±���
| �� �� | �� NaF | �� NaI | �� MgO |
| ���ӵ���� | 1 | 1 | 2 |
| ����(10-10m) | 2.31 | 3.18 | 2.10 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
���и����У����ɡ����ۼ������ϵ����ȷ����
| ѡ�� | ���� | ���� |
| A | ���ܣ�N��N��Cl��Cl | ���ʷе㣺N2��Cl2 |
| B | �����пɵ����H+������H2SO4��CH3COOH | ���ԣ�H2SO4��CH3COOH |
| C | Ԫ�صķǽ����ԣ�N��P | ���ԣ�HNO3��H3PO4 |
| D | �����ԣ�Fe3+��Cu2+ | ��ԭ�ԣ�Fe2+��Cu |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
[���ʽṹ������]��15�֣�
��֪X��Y��Z����Ԫ�ص�ԭ������֮�͵���48��X��һ��1:1�ͳ�����̬�⻯������м��ЦҼ����Цм���Z�ǽ���Ԫ�أ�Z�ĵ��ʺͻ������й㷺����;����֪Z�ĺ˵����С��28���Ҵ������2��δ�ɶԵ��ӡ���ҵ������ZO2��̼�ᱵ������״̬����ȡ������M��M�ɿ���һ�ֺ������Σ���M�������ġ�ѹ�����ܡ���Ӧ���ڳ������ķ���װ�á���X���߷�����M�������С�ظ���λΪ�����壨����ͼ��������λ��ΪZ4����ռ������λ��ΪBa2����ռ����������λ��ΪO2-��ռ��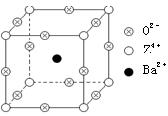
��1��Y�����ڱ���λ��______________��Z4���ĺ�������Ų�ʽΪ______________��
��2��X�ĸ����⻯����ӹ���Ϊ___________��X�ڸ��⻯������___________��ʽ�ӻ���X��Y�γɵĻ�������۵�Ӧ��_____������ڡ��� �����ڡ���X�ĸ��⻯����۵㡣
��3�����Ʊ�M�Ļ�ѧ��Ӧ����ʽ��________________________________________��
����M�����У�����Z4����������������ģ�Ba2������������Ķ��㣬��O2�������������______��
����M�����У�Z4��������λ��Ϊ________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��.��֪���н������壺Na��Po��K��Fe��Cu��Mg��Zn��Au����ѻ���ʽΪ��
��1���������ѻ�����____________________________________________��
��2�����͵���______________________________________________________��
��3��þ�͵���______________________________________________________��
��4��ͭ�͵���_____________________________________________________��
��.A��B��C��D���Ƕ�����Ԫ�أ�ԭ�Ӱ뾶D>C>A>B����֪��A��B����ͬһ���ڣ�A��C����ͬһ���壻Cԭ�Ӻ��ڵ�����������A��Bԭ�Ӻ��ڵ�������֮�ͣ�Cԭ��������������Dԭ��������������4����
�Իش�
��1��������Ԫ�طֱ��ǣ�A______��B______��C______��D______����Ԫ�����ƣ���
��2��������Ԫ�ص��ʵ��۵��ɸߵ��͵�˳����________����Ԫ�����ƣ���
��3��C�Ĺ�̬��������________���壬D�Ĺ�̬������________���塣
��4��д��A��B��D��ɵĻ�������B��C��ɵĻ��������Ӧ�Ļ�ѧ����ʽ_______________________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
ij��λ������Ϊ����ɫ���壬��ԭ��������С�����A��B��C��D��E����Ԫ�ع��ɣ���ԭ�Ӹ�����Ϊ14:4:5:1:1������C��DԪ��ͬ������ԭ������DΪC�Ķ�����EԪ�ص���Χ�����Ų�Ϊ��n��l��dn+6nsl���ش��������⡣
��1������λ������Ļ�ѧʽΪ____ ��Ԫ��B��C��D�ĵ�һ�������ɴ�С������˳��Ϊ �� ����Ԫ�ط��ű�ʾ��
��2��DԪ��ԭ�ӵ����������Ų�ͼΪ ��DC42�������幹��Ϊ____ ��
��3��AԪ����EԪ�ؿ��γ�һ�ֺ�ɫ������侧��ṹ��Ԫ��ͼ����û�����Ļ�ѧʽΪ �û��������������ȼ�գ�����һ���ػ�ɫ�����һ�����壬д���÷�Ӧ�Ļ�ѧ����ʽ ��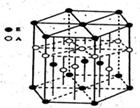
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
��֪A��B��C��D�������壬��ӦA+B  C+D�������仯��ͼ��ʾ������˵����ȷ����
C+D�������仯��ͼ��ʾ������˵����ȷ����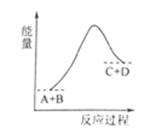
A����A������Ӧ������C���淴Ӧ������ͬʱ����ƽ��״̬��������
B����A��B��C��D��Ũ����ͬʱ����Ӧ����ƽ��״̬
C����Ӧ����ܼ�����������������ܼ��ܡ�������
D���÷�Ӧ�Ƿ��ȷ�Ӧ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
��֪��
(1)����ʧˮ���Ȼ�ѧ����ʽΪCuSO4��5H2O(s)===CuSO4(s)��5H2O(l) ��H����Q1 kJ/mol
(2)�����£���ˮ����ͭ����ˮ���Ȼ�ѧ����ʽΪCuSO4(s)===Cu2��(aq)��SO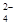 (aq) ��H��
(aq) ��H��
��Q2 kJ/mol
(3)����(CuSO4��5H2O)����ˮʱ��Һ�¶Ƚ��͡���Q1��Q2�Ĺ�ϵ��(Q1��Q2Ϊ����) �� ��
| A��Q1>Q2 | B��Q1��Q2 | C��Q1<Q2 | D����ȷ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
���о��尴A1�ͷ�ʽ���н��ܶѻ����ǣ� ����
| A���ɱ���NaCl������ͭ |
| B��ZnS������þ�������� |
| C��ˮ�������ʯ������� |
| D��ZnS��NaCl������þ |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com