
������Ҫ�Ļ�����Ʒ�ͻ���ԭ�ϡ�
��1�����ĵ���ʽ�� ��
��2����֪��

�ٺϳɰ����Ȼ�ѧ����ʽ�� ��
�ڽ����¶ȣ��÷�Ӧ�Ļ�ѧƽ�ⳣ��K �������������С�������䡱����
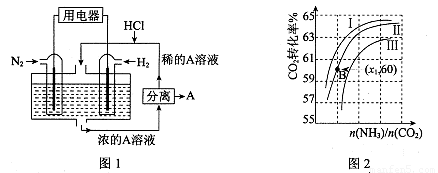
��3������������N2��H2Ϊ��Ӧ�������A��ϡ����Ϊ�������Һ��������������ṩ���ܣ����̵ܹ�������ȼ�ϵ�أ�װ����ͼl��ʾ��
��������ĵ缫��Ӧʽ�� ��A�� ��
��4���ð��ϳ����صķ�ӦΪ2NH3(g)+CO2(g) CO(NH2)2��l��+ H2O(g)����ҵ����ʱ��ԭ��������ˮ������ͼ2��ʾCO2��ת�����백̼��
CO(NH2)2��l��+ H2O(g)����ҵ����ʱ��ԭ��������ˮ������ͼ2��ʾCO2��ת�����백̼�� ��ˮ̼��
��ˮ̼��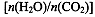 �ı仯��ϵ��
�ı仯��ϵ��
������I��II��III��Ӧ��ˮ̼�������� ��
�ڲ��B�㰱��ת����Ϊ40%����x1 ��
(13��)
��1�� ��2�֣�
��2�֣�
��2����N2(g)+3H2(g)=2NH3(g) ��H=-92kJ/mol
������
��3��N2+8H++6e-=2NH4+ (2��) NH4Cl ��2�֣�
��4���� (1��)�� 3 ��2�֣�
��������
�����������1�����ķ����е�����ԭ��֮���γ�һ�Թ��õ��Ӷԣ������ĵ���ʽ��
��2���ٸ��ݷ�Ӧ�ġ�H=��Ӧ����ܼ���-��������ܼ��ܣ�����ϳɰ��ġ�H=946kJ/mol+3��436kJ/mol-3��391��2kJ/mol=-92kJ/mol�����Ժϳɰ����Ȼ�ѧ����ʽ��N2(g)+3H2(g)=2NH3(g) ��H=-92kJ/mol��
�ڸ÷�Ӧ���ȣ����Խ��£�ƽ�������ƶ���������Ũ������Ӧ��Ũ�ȼ�С����ѧƽ�ⳣ��������
��3���õ�صı��ʷ�Ӧ�Ǻϳɰ���Ӧ�����������ǵ���������ԭ��Ӧ���缫��ӦʽΪN2+8H++6e-=2NH4+�����ɵ�笠��������Ȼ����������Ȼ�泥�����A��NH4Cl��
��4����̼��һ��ʱ��ˮ̼��Խ��˵��ԭ�����к�������̼Խ�٣�������̼��ת����Խ�ͣ����Զ�����̼ת������͵ļ�Ϊˮ̼�����ģ��Ǣ����ߣ�B�������̼��ת������60%��������ת������40%����NH3��CO2����ʼ���ʵ����ֱ�Ϊn1��n2����n1��40%/2= n2��60%,���n1/n2= x1=3��
���㣺�������ʽ���Ȼ�ѧ����ʽ���缫��Ӧʽ����д��ƽ���ƶ����жϣ���ѧƽ�ⳣ�����жϣ���ͼ��ķ�������


 ��У����ϵ�д�
��У����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| 3 | 4 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013��ӱ�ʡ��ɽһ�и����ڶ����¿���ѧ�Ծ����������� ���ͣ������
(14��)������Ҫ�Ļ�����Ʒ֮һ���о��ϳɰ���Ӧ������Ҫ���塣
(1) ��֪�������л�ѧ����Ҫ���յ������ֱ�Ϊ��
 ��
��
д����N2��H2Ϊԭ�Ϻϳ�NH3���Ȼ�ѧ����ʽ________________________��
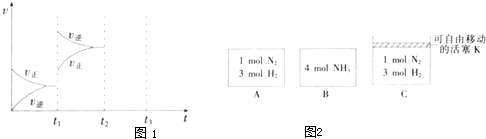
(2) ijС���о���������������ʱ���ı�ijһ������������Ӧ��Ӱ�죬ʵ��������ͼ��ʾ��
��t1ʱ�̸ı������Ϊ__________________��
��t2ʱ�̣���ѹ���뺤��,t3ʱ�̴ﵽƽ�⡣��ͼ�л���t2ʱ�̺�����ʱ仯ͼ��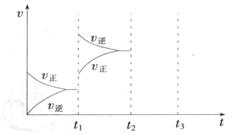
(3) ��ͬ�¶��£�A��B��C�����ܱ�������A��B���ݣ�C���п������ƶ��Ļ���K���������г�����ͼ��ʾ��Ӧ���ʼʱ���ƻ���Kʹ���������ȣ�һ��ʱ�����ﵽƽ�⡣
�ٴﵽƽ��ʱ��A��C����������NH3��Ũ�ȷֱ�Ϊcl��c2����c1______c2(�>������<����=��)��
�ڴﵽƽ��ʱ����A��B�������з�Ӧ���ת���ʷֱ�Ϊ��(A),��(B)�����(A)+��(B)______1(� >������<����=������
�۴ﵽƽ��ʱ��������C���������ʼʱ��3/4����ƽ��ʱ����C��H2���������Ϊ_______��
(4) ֱ�ӹ���ʽ����ȼ�ϵ�أ�DAFC)����KOH��ҺΪ�������Һ�����ط�ӦΪ 4NH3+3O2=2N2+6H2O�����ĵ缫��ӦʽΪ__________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012��ӱ�ʡʯ��ׯ�и����ڶ���ģ�������ۺϻ�ѧ�Ծ����������� ���ͣ������
(14��)������Ҫ�Ļ�����Ʒ֮һ���о��ϳɰ���Ӧ������Ҫ���塣
(1) ��֪�������л�ѧ����Ҫ���յ������ֱ�Ϊ��
 ��д����N2(g)��H2(g)Ϊԭ�Ϻϳ�NH3(g)���Ȼ�ѧ����ʽ_______________
��д����N2(g)��H2(g)Ϊԭ�Ϻϳ�NH3(g)���Ȼ�ѧ����ʽ_______________
(2) ijС���о���������������ʱ���ı�ijһ������������Ӧ��Ӱ�죬ʵ��������ͼ��ʾ��
��t1ʱ�̸ı������Ϊ__________________
��t2ʱ�̣���ѹ���뺤��,t3ʱ�̴ﵽƽ�⡣��ͼ�л���t2ʱ�̺�����ʱ仯ͼ��
(3) ��ͬ�¶��£�A��B��C�����ܱ�������A��B���ݣ�C���п������ƶ��Ļ���K���������г�����ͼ��ʾ��Ӧ���ʼʱ���ƻ���K��ʹ���������ȣ�һ��ʱ�����ﵽƽ�⡣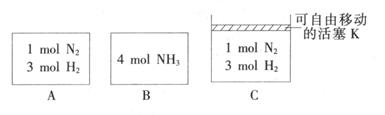
�ٴﵽƽ��ʱ��A��C����������NH3��Ũ�ȷֱ�Ϊcl��c2����c1______c2(�>������<����=��)��
�ڴﵽƽ��ʱ����A��B�������з�Ӧ���ת���ʷֱ�Ϊ ����
����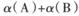 ______1(� >������<����=������
______1(� >������<����=������
�۴ﵽƽ��ʱ��������C���������ʼʱ�� ����ƽ��ʱ����C��H2���������Ϊ_______
����ƽ��ʱ����C��H2���������Ϊ_______
(4) ֱ�ӹ���ʽ����ȼ�ϵ�أ�DAFC)����KOH��ҺΪ�������Һ�����ط�ӦΪ 4NH3+3O2=2N2+6H2O�����ĵ缫��ӦʽΪ__________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ��ӱ�ʡ�����ڶ����¿���ѧ�Ծ��������棩 ���ͣ������
(14��)������Ҫ�Ļ�����Ʒ֮һ���о��ϳɰ���Ӧ������Ҫ���塣
(1) ��֪�������л�ѧ����Ҫ���յ������ֱ�Ϊ��
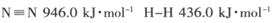
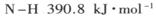 ��
��
д����N2��H2Ϊԭ�Ϻϳ�NH3���Ȼ�ѧ����ʽ________________________��
(2) ijС���о���������������ʱ���ı�ijһ������������Ӧ��Ӱ�죬ʵ��������ͼ��ʾ��
��t1ʱ�̸ı������Ϊ__________________��
��t2ʱ�̣���ѹ���뺤��,t3ʱ�̴ﵽƽ�⡣��ͼ�л���t2ʱ�̺�����ʱ仯ͼ��

(3) ��ͬ�¶��£�A��B��C�����ܱ�������A��B���ݣ�C���п������ƶ��Ļ���K���������г�����ͼ��ʾ��Ӧ���ʼʱ���ƻ���Kʹ���������ȣ�һ��ʱ�����ﵽƽ�⡣

�ٴﵽƽ��ʱ��A��C����������NH3��Ũ�ȷֱ�Ϊcl��c2����c1______c2(�>������<����=��)��
�ڴﵽƽ��ʱ����A��B�������з�Ӧ���ת���ʷֱ�Ϊ��(A),��(B)�����(A)+��(B)______1(� >������<����=������
�۴ﵽƽ��ʱ��������C���������ʼʱ��3/4����ƽ��ʱ����C��H2���������Ϊ_______��
(4) ֱ�ӹ���ʽ����ȼ�ϵ�أ�DAFC)����KOH��ҺΪ�������Һ�����ط�ӦΪ 4NH3+3O2=2N2+6H2O�����ĵ缫��ӦʽΪ__________________��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com