
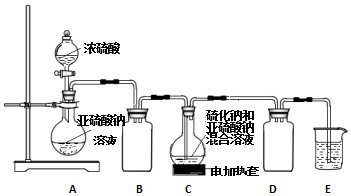
���� ��1��Aװ��������������Ũ�����Ʊ������������壻Cװ���ж������������Ʒ�Ӧ����S�����Ʊ�Na2S2O3��
��2��Bװ�÷�ֹ��������������������ֽ⣬SO2���ܹ�����
��3����������ƣ�Na2S2O3�������ֽ⣺S2O32-+2H+�TH2O+SO2��+S��������̼���ƣ�CO32-+2H+�TH2O+CO2��A��ӦΪ����Һ�����ų���������ĸ��ţ������ȳ�������������֤������̼�����ɣ��ݴ˷�����
��4�����������������ƹ�ҵ��Ʒ�к���Na2SO3����Na2S2O3ϡ��Һ��BaCl2��Һ����������ɣ�Na2SO3��BaCl2��Һ����������ᱵ��ɫ�������ɣ��������Ȼ�����Һ����������м�ϡ���ᣬ���ɵ�������Ʒ����Һ���鼴�ɣ�
��� �⣺��1��Aװ��������������Ũ�����Ʊ������������壬�����ķ�ӦΪ��Na2SO3+H2SO4��Ũ��=Na2SO4+H2O+SO2����Cװ���ж������������Ʒ�Ӧ��Na2S+H2O+SO2=Na2SO3+H2S��2H2S+SO2=3S��+2H2O��S����ʹ��Һ���ǣ�S��s��+Na2SO3��aq��$\frac{\underline{\;��\;}}{\;}$Na2S2O3��aq������Һ���壻
�ʴ�Ϊ��Na2SO3+H2SO4��Ũ��=Na2SO4+H2O+SO2���� S��
��2��Bװ�÷�ֹ�����������֪����������������ֽ⣬SO2��������Һ�����ԣ�����ֽ⣻
�ʴ�Ϊ����ֹ��Һ��������SO2��������Һ�����ԣ������ֽ⣻
��3������������ƣ�Na2S2O3�������ֽ⣺S2O32-+2H+�TH2O+SO2��+S��������̼���ƣ�CO32-+2H+�TH2O+CO2��A��ӦΪ����Һ�����ų���������ĸ��ţ����������л�ԭ�ԣ�����ѡ�����Ը��������Һ��ȥ�����ö��������Ư���ԣ���Ʒ����Һ������������Ƿ������������ó���ʯ��ˮ��֤������̼�����ɣ�
�ʴ�Ϊ��ADCB��Ʒ����Һ��
��֤�������к���̼���Ƶ�ʵ��������װ��C��Ʒ����Һ����ɫ��B�г���ʯ��ˮ����ǣ�
�ʴ�Ϊ��װ��C��Ʒ����Һ����ɫ��B�г���ʯ��ˮ����ǣ�
��4�����������������ƹ�ҵ��Ʒ�к���Na2SO3�����ȼ�ˮ���ϡ��Һ��Na2S2O3ϡ��Һ��BaCl2��Һ����������ɣ�Na2SO3��BaCl2��Һ����������ᱵ��ɫ�������ɣ��������Ȼ�����Һ����������м�ϡ���ᣬ���ɵ�������Ʒ����Һ���鼴�ɣ�
�ʴ�Ϊ���Ȼ�����Һ�����ᣮ
���� ���⿼�����ʵ��Ʊ�ʵ�顢ʵ�鷽����ƣ�Ϊ��Ƶ���㣬������ѧ���ķ���������ʵ�������Ŀ��飬�Ѷ��еȣ���ȷʵ��ԭ���ǽⱾ��ؼ����������ʵ����ʷ������ע��Ԫ�ػ�����֪ʶ�Ļ��ۺ�������ã�



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
| A�� | ��ѿ�Ǻ��� | B�� | ���Ǻ���ѿ�� | C�� | ���ۺ���ά�� | D�� | ���Ǻ������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | 62g���ף�����P4Ϊ��������ṹPԭ��λ�ڶ��㣩�к�P-P���ۼ�Ϊ6NA | |
| B�� | 1molCl2ȫ��������Ӧʱ������ʲôʱ���ǵõ��ĵ�����һ��Ϊ2NA | |
| C�� | ��һ��CO������Ϊa g����CO��Ħ������Ϊa NA | |
| D�� | ������10LpH=13��NaOH��Һ�к��е�OH-������ΪNA |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �ڱ�״���£�NA��H2O ������ռ���ԼΪ22.4L | |
| B�� | 0.1 mol H2��0.2 mol O2��0.3 mol N2��0.4 mol CO2��ɵĻ�������ڱ�״���µ����ԼΪ22.4 L | |
| C�� | ���³�ѹ�£�2.24 L CO��CO2��������к��е�̼ԭ����ĿΪ0.1 NA | |
| D�� | 0.5mol/L��NaCl��Һ��Cl-�ĸ���Ϊ��0.5NA |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
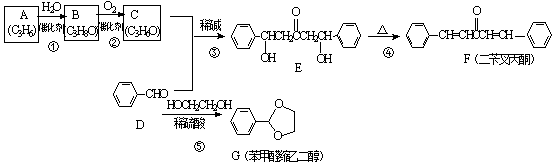
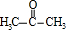 ��A�Ӿ۲���Ľṹ��ʽ��
��A�Ӿ۲���Ľṹ��ʽ�� 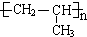 ��
��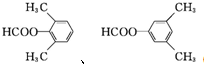 ��
��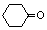 ������ͪ���ϳ�
������ͪ���ϳ� 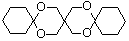 [��֪HCHO������ȩ�л�ԭ����ǿ�ģ�����Ca��OH��2������]
[��֪HCHO������ȩ�л�ԭ����ǿ�ģ�����Ca��OH��2������]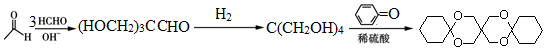 ��
���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
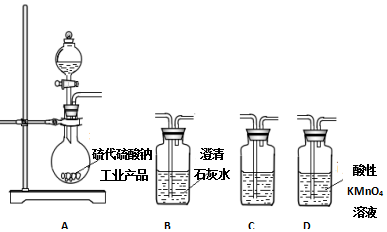
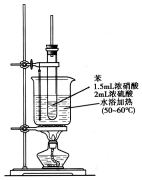 ����������һ�־��п�������ζ����ɫ��״Һ�壬�ܶȱ�ˮ��������Ⱦ�ϵ���Ҫԭ�ϣ�ʵ��������ͼ��ʾ��װ������ȡ��
����������һ�־��п�������ζ����ɫ��״Һ�壬�ܶȱ�ˮ��������Ⱦ�ϵ���Ҫԭ�ϣ�ʵ��������ͼ��ʾ��װ������ȡ��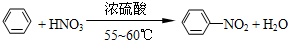 ��
��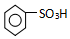 ���������������IJ����½���д����������Ӧ�Ļ�ѧ����ʽ
���������������IJ����½���д����������Ӧ�Ļ�ѧ����ʽ +H2SO4��Ũ��$\stackrel{��}{��}$
+H2SO4��Ũ��$\stackrel{��}{��}$ +H2O��
+H2O���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
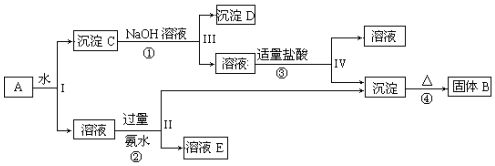
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
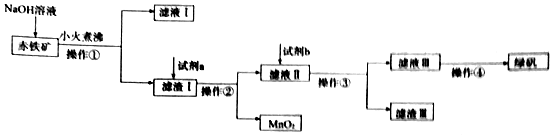
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | 0.75��2.2 | B�� | 1.5��2.2 | C�� | 0.75��4.4 | D�� | 1.5��4.4 |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com