
����Ŀ����.���з�Ӧ��CO(g)��H2O(g)![]() CO2(g)��H2(g) ��H<0����850��ʱ��K��1��
CO2(g)��H2(g) ��H<0����850��ʱ��K��1��
��1���������¶ȵ�950��ʱ���ﵽƽ��ʱK__________1(����ڡ���С�ڡ����ڡ�)��
��2��850��ʱ������һ�ݻ��ɱ���ܱ�������ͬʱ����1.0 mol CO��3.0 mol H2O��1.0 mol CO2��x mol H2����
�ٵ�x��5.0ʱ������ƽ����_____________(�����Ӧ�����淴Ӧ��)�����ƶ���
����Ҫʹ������Ӧ��ʼʱ������Ӧ������У���xӦ�����������_______________��
��.��һ�̶��ݻ����ܱ�������,����һ���¶�,��һ�������·������·�Ӧ:2A(g)+B(g)![]() 3C(g),��֪����1 mol A��2 mol B�Ҵﵽƽ���,������a mol C��
3C(g),��֪����1 mol A��2 mol B�Ҵﵽƽ���,������a mol C��
��3���ﵽƽ��ʱ,C�ڷ�Ӧ��������е����������___(�ú�a�Ĵ���ʽ��ʾ)��
��4������ͬ��ʵ��������,����ͬһ�����и�Ϊ����2 mol A��4 mol B,�ﵽƽ���,C�����ʵ���Ϊ___mol(�ú�a�Ĵ���ʽ��ʾ)����ʱC�ڷ�Ӧ��������е����������ԭƽ�����___(��������������С������������)��
��5������ͬʵ��������,����ͬһ�����и�Ϊ����2 mol A��5 mol B,��Ҫ��ƽ���C�ڷ�Ӧ��������е������������ԭƽ����ͬ,��Ӧ����___mol C��
���𰸡�С�� �淴Ӧ x��3.0 ![]() 2a ���� 1
2a ���� 1
��������
��1��CO(g)��H2O(g)![]() CO2(g)��H2(g) ��H<0������Ӧ���ȣ��������¶ȣ�ƽ�������ƶ��������¶ȵ�950��ʱ�� KС��1��
CO2(g)��H2(g) ��H<0������Ӧ���ȣ��������¶ȣ�ƽ�������ƶ��������¶ȵ�950��ʱ�� KС��1��
��2����850��ʱ������һ�ݻ��ɱ���ܱ�������ͬʱ����1.0 mol CO��3.0 mol H2O��1.0 mol CO2��0.5 mol H2��Q=![]() >K=1������ƽ�����淴Ӧ�����ƶ���
>K=1������ƽ�����淴Ӧ�����ƶ���
����Ҫʹ������Ӧ��ʼʱ������Ӧ������У���������Q<K��Q=![]() <K=1��x��3.0��
<K=1��x��3.0��
��3����Ӧǰ���������ʵ������䣬���Է�Ӧ������������ʵ���Ϊ3mol���ﵽƽ��ʱ��C�ڷ�Ӧ��������е����������![]() ��
��
��4����Ӧǰ���������ʵ������䣬����2 mol A��4 mol B�����1 mol A��2 mol BΪ��Чƽ�⣻�ﵽƽ���,C�����ʵ���Ϊ2amol����ʱC�ڷ�Ӧ��������е����������ԭƽ����Ȳ��䣻
��5����Ӧǰ���������ʵ������䣬Ͷ�ϱ���ͬΪ��Чƽ�⣬��Ӧ����xmol C���� ����x=1mol��
����x=1mol��


 ���ѵ����Ԫ��ĩ���100��ϵ�д�
���ѵ����Ԫ��ĩ���100��ϵ�д� ��˼άС�ھ�100����ҵ��ϵ�д�
��˼άС�ھ�100����ҵ��ϵ�д� ��ʦָ��һ��ͨϵ�д�
��ʦָ��һ��ͨϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��A��B��C��D��E��F��������������ת����ϵ��EΪ����ɫ��ĩ��ͨ�����ں����������Ϊ��������Դ��

��1��A��C��E��F�Ļ�ѧʽ��
A______��C______��E______��F______��
��2���͵�C����Һ��ͨ������CO2������ֵ�������_____���йط�Ӧ�Ļ�ѧ����ʽΪ____��
��3������C��F��ҺӦѡ�õ��Լ���_____���йط�Ӧ�����ӷ���ʽΪ_____��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����ڷ�ӦA(g)+3B(g)![]() 2C(g)�����и����ݱ�ʾ��ͬ�����µķ�Ӧ���ʣ����з�Ӧ���е��������� ��
2C(g)�����и����ݱ�ʾ��ͬ�����µķ�Ӧ���ʣ����з�Ӧ���е��������� ��
A��v(A)=0.01 mol/(Ls) B��v(C)=1.0mol/(Lmin)
C��v(B)=0.60mol/(Lmin) D��v(B)=0.04 mol/(Ls)
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��(1)����ͨ��Fe(OH)3���壬�ɿ���������ͨ·�����������__________________
(2)19gij���۽������Ȼ���ACl2�к���0.4mol Cl�����ӣ���ACl2��Ħ������________
(3)�ڱ�״���£����4.2g����A�������3360mL����A������ʲô����________
(4)�ڱ�״����15 g CO��CO2�Ļ�����壬���Ϊ11.2L����CO2��CO�����֮����_____________
(5)�ڱ�״���£���224 L HCl��������635 mLˮ(ˮ���ܶȿ���1g/mL)�У�����������ܶ�Ϊ1.18 g��cm��3�������������ʵ���Ũ����___________
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��������ƽ������ƿ����Ͳ�dz�������ѧ��ѧ������������֪��Щ������ʹ���Ƕ����о���ѧ�Ļ�����
(1)���в����У�����ƿ�����߱��Ĺ�����________________ (�����)��
A.����һ�����ȷŨ�ȵı���Һ B.������Һ C.��������ƿ������µ������������Һ D.ȷϡ��ijһŨ�ȵ���Һ E.��ȡһ�������Һ�� F.���������ܽ��������
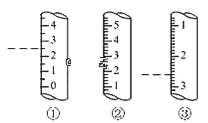
(2)��ͼ��ijЩ�����Ŀ̶Ȳ���ʾ��ͼ��ͼ�и���������Ϊ��ʾ����������Ϊ��Ͳ����_______ (����)������Ϊ_____mL��
(3)ʵ������98%��Ũ��������(Ũ�������1.84g/mL)450mL 0.1 mol��L-1�����ᡣ�ش��������⣺
��������Ӧѡ�õIJ�����������Ͳ���ձ���������������___________��Ӧ��ȡ________mL 98%��Ũ���ᡣ
�ڶ�Ũ����ϡ�������������������ʹ�����Ƶ�����Ũ�ȴ���0.1mol/L��_____(�����)
A.δ����ȴ���Ƚ���Һע������ƿ��
B.ҡ�Ⱥ���Һ����ڿ̶��ߣ��ټ�ˮ���̶���
C.����ƿ��ԭ����������ˮ
D.����ʱ���ӹ۲�Һ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��̼�Ȼ�ԭ���㷺���ںϽ��ϵ��Ʊ����ش���������
(1) һ���Ʊ����������ķ�Ӧԭ��Ϊ23Al2O3+ 15C+5N2=2Al23O27N5+15CO,����Al23O27N5 �е��Ļ��ϼ�Ϊ_____���÷�Ӧ��ÿ���� l mol Al23O27N5ת�Ƶĵ�����Ϊ_______________ ��
(2) ���̼��ұ���������ܶ෴Ӧ�����е�������Ӧ���£�
Al2O3 (s) +3C(s)=Al2OC(s) +2CO(g) ��H1
2Al2OC(s)+3C(s)=Al4C3(s)+2CO(g) ��H2
2Al2O3(s) +9C(s)= Al4C3(s)+6CO(g) ��H3
�١�H3=________ ( �á�H1����H2��ʾ)��
��Al4C3�����������ᷴӦ�Ʊ��������÷�Ӧ�Ļ�ѧ����ʽΪ____________��
(3)������̼�Ȼ�ԭ���̺Ͻ��������Ӧ ��CO��CO2ƽ���ѹ�ȵ���Ȼ����(![]() ) ���¶ȵĹ�ϵ��ͼ��ʾ(��֪Kp ����ƽ���ѹ����Ũ�ȼ������õ�ƽ�ⳣ������ѹ����ѹ �� ��������ʵ�������)��
) ���¶ȵĹ�ϵ��ͼ��ʾ(��֪Kp ����ƽ���ѹ����Ũ�ȼ������õ�ƽ�ⳣ������ѹ����ѹ �� ��������ʵ�������)��

I. Mn3C(s)+4CO2(g) 3MnO(s)+ 5CO(g) Kp(I)
II. Mn(s) +CO2(g) MnO(s) +CO(g) Kp (II)
III. Mn3C(s)+CO2(g) 3Mn(s) +2CO(g) Kp(III)
�١�H>O�� ��Ӧ��_________ (�I ����II�� ��III��)��
��1200Kʱ�� һ���Ϊ 2L �ĺ����ܱ���������17.7gMn3C( s)��0.4molCO2 ��ֻ������ӦI��5min��ﵽƽ�⣬��ʱCO��Ũ��Ϊ0.125mol/L����0��5 min�� v(CO2)=_______��
����һ����ɱ���ܱ������м���һ������Mn(s )������һ������CO2(g) .ֻ������Ӧ
II��������˵����ӦII �ﵽƽ��״̬����________ (����)��
A. ������������ٸı�
B.������������ٸı�
C.��������������ٸı�
��������ܱ������м���Mn3C������0.l molCO2, ��ֻ������ӦIII. ����A�㷴Ӧ�ﵽƽ��ʱ����������ѹΪakPa, CO2��ת����Ϊ______; A���Ӧ�¶��µ�Kp(III) =______��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ѧ�ϳ���AG��ʾ��Һ�����ȣ�AG=![]() ��25������0.100mol��L-1NaOHҺ�ζ�20.00 mL 0.100 mol��L-1 HNO2��Һ�� AG������NaOH��Һ�����(V)��ϵ��ͼ��ʾ������˵������ȷ����
��25������0.100mol��L-1NaOHҺ�ζ�20.00 mL 0.100 mol��L-1 HNO2��Һ�� AG������NaOH��Һ�����(V)��ϵ��ͼ��ʾ������˵������ȷ����
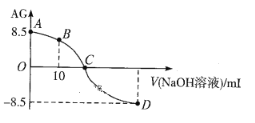
A. D����Һ�е�pH=11.25
B. B����Һ�д���2c(H+)��2c(OH-)=c(NO2-)��c(HNO2)
C. C��ʱ������NaOH��Һ�����С��20.00 mL
D. 25��ʱ��HNO2�ĵ���ƽ�ⳣ��Ka=1.0��10-5.5
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ij���жԴ������м�⣬���ָ�����Ҫ��Ⱦ��Ϊ�����������PM2.5(ֱ��С�ڵ���2.5��m������������)������Ҫ��ԴΪȼú��������β���ȡ���ˣ���PM2.5��SO2��NOx�Ƚ����о�������Ҫ���塣��ش��������⣺
(1)PM2.5��ɢ�ڿ������γɵķ�ɢϵ________(����ڡ������ڡ�)���塣
(2)��PM2.5����������ˮ�����Ƴɴ�������������ø���������ˮ���������ӵĻ�ѧ��ּ���ƽ��Ũ�����±���
���� | K�� | Na�� | NH4+ | SO42- | NO3- | Cl�� |
Ũ��/ mol��L��1 | 4��10��6 | 6��10��6 | 2��10��5 | 4��10��5 | 3��10��5 | 2��10��5 |
���ݱ��������жϴ�������Ϊ________(��ᡱ�)�ԣ���ʾ����������Ե�c(H��)��c(OH��)��________mol��L��1��
(3)Ϊ����SO2���ŷţ�����ijЩ��Һϴ�Ӻ�SO2���������������ʿ���ϴ�Ӽ�����__________________________(����ĸ)��
a.Ca(OH)2����b.Na2CO3����c.CaCl2����d.NaHSO3
(4)����β����NOx��CO�����ɼ�ת����
�����������������¶�Խ�ߣ���λʱ����NO�ŷ���Խ��д������������NO�Ļ�ѧ����ʽ��_____________________________��
������ȼ�Ͳ���ȫȼ��ʱ����CO��Ŀǰ��������β��ϵͳ��װ�ô�ת�����ɼ���CO��NO����Ⱦ���仯ѧ��Ӧ����ʽΪ__________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���鼰�仯������ұ��ҽ�ơ����������о�����Ҫ���á�������Ȼ���еĺ������٣�������������Ϳ������ʽ���ڡ��Ը�ѡ���Ļ������Ҫ�ɷ���Bi2S3����������Bi2O3��SiO2��Cu2S��FeS2�����ʣ�ͨ�������������͵�������������������̣�

��֪��I.���Ȼ������������У��������μ���NaC1O3���Է�����Cl2;
II.BiCl3����ˮ�����ɲ����Ե�BiOCl����������Ũ�����м�����ˮ��;
III.�����ԣ�Fe3+��Cu2+��Bi3+��H+.
��ش��������⣺
��1�����Ȼ�������ʱ��Ϊ����߽�ȡ���ʣ��ɲ�ȡ�Ĵ�ʩ��________________����дһ��������������������ҪĿ����______________________.
��2���������к���S��____________��д��ѧʽ��������Һ�������Ľ�����������Na+��Bi3+��___________________.
��3��д�����Ȼ���������Bi2S3��������Ӧ�����ӷ���ʽ__________________________.
��4������ԭ����������������Ӧ�����ӷ���ʽΪ2Bi+3Cu2+=2Bi3++3Cu��____________��
��5������⡱���̵ļ���װ����ͼ��ʾ��װ����NΪ��Դ��______________�����������������������������Ϸ�������Ҫ�缫��ӦʽΪ__________________________��

�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com