
�±���Ԫ�����ڱ�����Ԫ�ص�һ���֣�������Ԫ��X����������ϼ��ǣ�5��Y�ĵ���
���ڿ�����ȼ�ա�
| W | X | Y |
| | | Z |
| ��� | �����Ʋ� | ��ѧ����ʽ |
| ʾ�� | ������ | H2ZO3��4HI=Z����2I2��3H2O |
| 1 | | |
| 2 | | |
 ���������������Բ��������ϵ�д�
���������������Բ��������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��16�֣�X��Y��Z��W�dz��������ֶ�����Ԫ�أ���ԭ���������������������Ϣ���±���
| Ԫ�� | �����Ϣ |
| X | X�Ļ�̬ԭ�����������Ų�ʽΪnsnnpn |
| Y | Y�ǿ����к�����ߵ�Ԫ�� |
| Z | Z�ǵؿ��к�����ߵĽ���Ԫ�� |
| W | W�ĵ����dz����İ뵼����� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�±���Ԫ�����ڱ���һ���֡��������е���ĸ�ֱ����ijһ��ѧԪ�ء�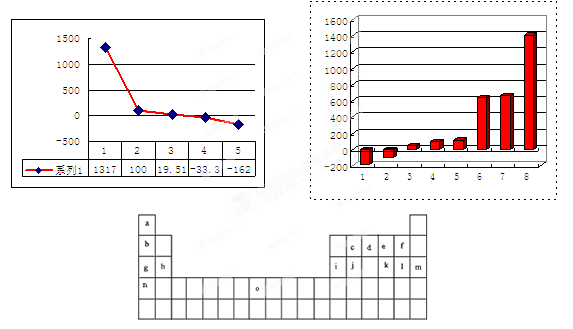
�Իش���������
��1��Ԫ�ء�O�������ڱ��е�λ���� ��
��2��������c���ļ����Ų�ʽ ��
��3����������8��Ԫ�ذ������۵��С˳�������ͼ���ϣ��������С�1������ ������ĸ����
��4��b��c��d��e��f���⻯��ķе㣨�棩ֱ������ͼ���ң����С�5���⻯��Ļ�ѧʽΪ�� �����С�1���⻯��ĵ���ʽΪ�� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��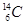 ��
��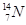 ��
�� ��
�� ��
��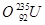 ��
��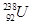 ��
�У�
��1�� �� ��Ϊͬλ��
��2�� �� �����������,�����ܻ���Ϊͬλ��
��3�� �� �����������,�������������,���Բ���ͬһ��Ԫ�أ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
A���л������Σ�B��C��D�dz�������� A��B��C��D��ɫ��Ӧ�ʻ�ɫ��ˮ��Һ���ʼ��ԣ�����B�ļ�����ǿ��X��Y��������������������塢����ϢϢ��أ����ǵľ���������ͬ��A��B�����ʵ�����Ӧ����D��һ�����嵥�ʣ�C���ȷֽ�õ�Y��D��X��B��C��Ӧ����D��X��E������Ԫ����ɣ�ʽ��Ϊ83����EͶ��X�еõ�B������Z��Z�ڱ�״���µ��ܶ�Ϊ0.76g��L-1��
��1��A�Ļ�ѧʽ�� ��Y�ĵ���ʽ�� ��
��2��X�ķе��ͬ����ͬ��������Ҫ�ߣ�ԭ���� ��
��3��д��E���������ᷴӦ�Ļ�ѧ����ʽ
��4��д����D�ı�����Һ�в���ͨY����C�����ӷ���ʽ ��
��5��A��һ����ҪӦ���Ǹ���2A ��P +H2���õ�P��P��Һ�е�������ͨ����CaCl2ʹ֮������������ȫ����ʱ����Һ��Ca2+�����ʵ���Ũ������Ϊ ��
������Ksp=2.3��10-9������Һ������Ũ�ȡ�10-5mol��L-1��������Ϊ��ȫ������
��6��ʵ���ҳ���P������HCl��Ӧ���õ��л�����Ũ���������¹��ȷֽ���ij��ԭ�����壬���ʵ��֤���ֽ�����л�ԭ������Ĵ��� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��ͼ��Ԫ�����ڱ���һ���֣���Ա��еĢ١�����Ԫ�أ���д���пո�
��1������10��Ԫ���У��ǽ�������ǿ���� ������ţ���
��2��Ԫ�آڢ����ԭ�Ӹ�����1:1:1�γɵĻ�����ĽṹʽΪ ��Ԫ�آ�����γɵ�18e��������ĵ���ʽ ��
��3���ڢ������Ԫ���γɵĻ�����M��ԭ�Ӹ�����Ϊ3:4:2����������Ϊ42��M�к��еĻ�ѧ�������� ��
��4���Ƚ�Ԫ�آߢ��γɵ���̬�⻯����ȶ��ԣ� �� ���û�ѧʽ��ʾ��
��5��Ԫ�آݵ�������������������Һ��Ӧ�����ӷ���ʽ ��
��6��Ԫ�آܺ͢��γɵĻ��������� ������õ���ʽ��ʾ���γɹ��� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
A��B��C��D��EΪԭ��������������Ķ�����Ԫ�أ���֪A��B��E 3��ԭ������㹲��11�����ӣ�����3��Ԫ�ص�����������ˮ�����������ܷ�����Ӧ�����κ�ˮ��CԪ�ص������������ȴ�����������4��DԪ��ԭ�Ӵ�����������������������3��
��1��д������Ԫ�ط��ţ�
A ��B ��C ��D ��E
��2��A��E��Ԫ�ؿ��γɻ�����õ���ʽ��ʾ�仯������γɹ��̣� ��
��3��д��A��B��Ԫ������������ˮ���ﷴӦ�����ӷ���ʽ�� ��
��4���Ƚ�C��D������������ˮ��������ԣ����û�ѧʽ��ʾ�� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
X��Y��Z��L��M��N����Ԫ�ص�ԭ��������������X��Y��Z��L����ɵ����ʵĻ���Ԫ�أ�M�Ƕ�����ԭ�Ӱ뾶����Ԫ�أ�N�ǵؿ��к�����ߵĽ���Ԫ�ء�
�û�ѧ����ش��������⣺
��1�� M��Ԫ�����ڱ��е�λ��Ϊ ������Ԫ�ص�ԭ�Ӱ뾶�Ӵ�С��˳����
��2��Z��X��Ԫ�ذ�ԭ����Ŀ��l��3���ɷ���A, A�ĵ���ʽΪ ��Y��L��Ԫ�ذ�ԭ����Ŀ��l��2���ɷ���B��B�������Ļ�ѧ������Ϊ ��
��3������se��������������Ԫ�أ���֪�ǽ����ԣ�34Se<L������ԭ�ӽṹ����ԭ�� ��
��4����Y��L��M���ɵ�������Һ����������ۣ��������ӷ���ʽ�Լ���Ҫ�����ֽ���ԭ�� ��
��5����ʯī���缫��NCl3��Һ�����Һ���е�⣬����������R��R���ȷֽ����ɻ�����Q��д���������Q��ȡN�ĵ缫����ʽ�������� �������� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
������������Ԫ�صĵ��������ݣ���λ��kJ��mol��1�����ش��������⡣
| Ԫ�ش��� | I1 | I2 | I3 | I4 |
| Q | 2 080 | 4 000 | 6 100 | 9 400 |
| R | 500 | 4 600 | 6 900 | 9 500 |
| S | 740 | 1 500 | 7 700 | 10 500 |
| T | 580 | 1 800 | 2 700 | 11 600 |
| U | 420 | 3 100 | 4 400 | 5 900 |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com