
����Ŀ��ʵ������ȡ����ͨ�������ַ�����
���ù��������������Ȼ�粒��ȣ�
���ڳ������ù�������������Ũ��ˮ��Ӧ��

(1)�������ȡװ��ͼ�У�������Ӧѡ��װ��__________(����A������B������ͬ)��������Ӧѡ��װ��__________��
(2)�����Ȼ�����������ƻ������ȡ�����ķ�Ӧ����ʽ��________________��
(3)����ȡ�����Ҫ���ﰱ����Ӧѡ�õĸ������________________������ĸ����
A��Ũ���ᡡ����B����ʯ�ҡ�����C������������
(4)���鼯��ƿ���Ƿ��ռ��������ķ�����__________________________��
���𰸡�A B 2NH4Cl��Ca(OH)2![]() CaCl2��2NH3����2H2O B ��ʪ��ĺ�ɫʯ����ֽ��������ƿ�ڣ�����ֽ������˵�������Ѽ���
CaCl2��2NH3����2H2O B ��ʪ��ĺ�ɫʯ����ֽ��������ƿ�ڣ�����ֽ������˵�������Ѽ���
��������
(1)�ù��������������Ȼ�粒����Ʊ��������ǹ����������Ʊ�����ķ�Ӧ���ڳ������ù�������������Ũ��ˮ��Ӧ�ǹ����Һ�岻�����Ʊ����壻
(2)��ȡNH3�ķ�ӦΪ����NH4Cl��Ca(OH)2��Ӧ�����Ȼ��ơ�������ˮ��
(3)�����Ǽ������壬���������Ը�������Ӧ�ü��Ը������
(4)�����Ǽ������壬����ʪ��ĺ�ɫʯ����ֽ����ɫ��
(1)�ù��������������Ȼ�粒����Ʊ��������ǹ����������Ʊ�����ķ�Ӧ��Ӧѡ��Aװ�ã��ڳ������ù�������������Ũ��ˮ��Ӧ�ǹ����Һ�岻�����Ʊ�������Ӧѡ��Bװ�ã�
(2)��ȡNH3�ķ�ӦΪ����NH4Cl��Ca(OH)2��Ӧ�����Ȼ��ơ�������ˮ����Ӧ�Ļ�ѧ����ʽΪ��2NH4Cl+Ca(OH)2 ![]() CaCl2+2NH3��+2H2O��
CaCl2+2NH3��+2H2O��
(3)A��Ũ����Ͱ����ᷴӦ��������泥����ܸ��ﰱ����A����
B�������������ƾ�����ʪ�ԣ����Ը��ﰱ����B��ȷ��
C�����������Ͱ���������Ӧ�����ܸ��ﰱ����C����
�ʺ���ѡ����B��
(4)��ʪ��ĺ�ɫʯ����ֽ��������ƿ�ڣ������ֽ��������֤���ǰ����Ѿ��ռ�����


 ��У��������ĩ��̾�ϵ�д�
��У��������ĩ��̾�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ijУ��ѧ��ȤС������ͼ��ʾ���̳�ȥAlCl3�к��е�Mg2����K���������ӣ��������ܼ���AlCl3����ʧ����ش��������⣺
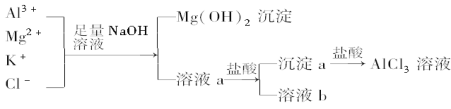
��1��д��������м�����������������Һʱ����Һ�з�����Ӧ�����ӷ���ʽ��________��________��________.����������Һ�ܷ��ð�ˮ���棬Ϊʲô��______________________.
��2����Һa�д��ڵ�������________������Һa�м�������ʱ�������Һ��pH��Ϊʲô��________��Ϊ�ˣ��Ľ�������____________________��
��3��Ϊ���о�AlCl3��������ʣ��ڵõ�AlCl3��Һ����εõ�AlCl3���壿____________________.
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ij�л���X�Ľṹ��ʽ��ͼ��ʾ�������й�˵������ȷ����
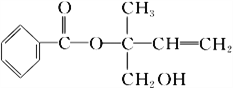
A. X�����к������ֹ�����
B. �������Ը��������Һ���𱽺�X
C. X��һ���������ܷ����ӳɡ��Ӿۡ�ȡ���������ȷ�Ӧ
D. �ڴ����������£�1 mol X�������5 mol H2�ӳ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��(1)���¡���ѹ�£��������O2��O3�������Ӹ�����______��������Ϊ_______��
(2)��֪16 g A��20 g Bǡ����ȫ��Ӧ����0.04 mol C��31.76 g D����C��Ħ������Ϊ_____��
(3)�������ܱ������зֱ����Ne��H2��O2�������壬�����ǵ��¶Ⱥ��ܶȶ���ͬʱ�������������ѹǿ��p���ֱ���p(Ne)��p(H2)��p(O2)��ʾ���ɴ�С��˳����____________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ij��ѧ����С������ʵ���ʾ��ͼ��ͼ��ʾ��ͼ�С�������ʾ�������� M��һ�ִ�������������壬YΪ��һ�����壬E���к���ɫ���������ʵ����������ֻ��������������ѡȡ��Na2CO3��Na2O2��NaCl��Na2O��CaCl2��(NH4)2CO3����ʯ�ҵȹ��弰����ˮ���ݴ�ʵ�飬���������գ�
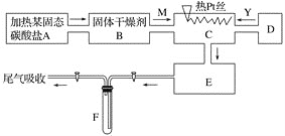
(1)A������װ�õ���Ҫ������ҩƷ��______________________��
(2)B����ѡ�ĸ������________����������______________________________��
(3)C�з�������Ҫ��Ӧ�Ļ�ѧ����ʽ��________________________________��
(4)��ȡY�����Dװ�����õ���Ҫ������__________________________��
��ȡY����Ļ�ѧ����ʽ��________________________��
(5)F�з�����Ӧ�Ļ�ѧ����ʽΪ__________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��H2��I2��һ���������ܷ�����Ӧ��H2(g)��I2(g) ![]() 2HI(g) ��H����a kJ/mol����֪��
2HI(g) ��H����a kJ/mol����֪��
![]() (a��b��c��������)��
(a��b��c��������)��
����˵����ȷ����
A��H2��I2��HI�����еĻ�ѧ�����ǷǼ��Թ��ۼ�
B���Ͽ�2 mol HI�����еĻ�ѧ����������ԼΪ(c��b��a) kJ
C����ͬ�����£�1 mol H2 (g)��1mol I2 (g)������С��2 mol HI (g)��������
D�����ܱ������м���2 mol H2 (g)��2 mol I2 (g)����ַ�Ӧ��ų�������Ϊ2a kJ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ij����С���ͬѧ������ͼ��ʾ��װ�ôӺ����Һ����H2O�⣬����I2��I-�ȣ��л��յ⡣�ش��������⣺

��1��װ��A�з�����Ӧ�����ӷ���ʽΪ________��
��2������X������Ϊ________��װ��D��������________��
��3��װ��C��������������ȴˮ��________���a����b�������룬��Ӧ�����ڽϵ��¶����ұ�����ҺpH =2���У�����Ҫԭ����________��
��4��������ƿ��Һ�徭���˵ôֵ⣬�ֵ�ɾ�________����������ƣ��õ����⡣
��5��Ϊ�ⶨij�����ˮ��I2�ĺ�����ȡ��l00mL��������ҺpH�μ�2�ε�����Һ��Ȼ����0.02500mol/LNa2S2O3����Һ�ζ������ı�Һ18.15mL����ζ��յ�ʱ������Ϊ________�������ˮ��I2�ĺ���=________mg/mL���������С�������λ����֪��I2 +2S2O32-=2I-+S4O62-����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��Ϊ��֤±�ص��������Ե����ǿ����ijС������ͼ��ʾװ�ý���ʵ��(�г���������ȥ���������Ѽ���)��

ʵ����̣�
��.���ɼУ�����a���μ�Ũ���ᡣ
��.��B��C�е���Һ����Ϊ��ɫʱ���н����ɼС�
��.��B����Һ�ɻ�ɫ��Ϊ�غ�ɫʱ���رջ���a��
��.����
��1����֤������������ǿ�ڵ��ʵ��������_________________________________________��
��2��B����Һ������Ӧ�����ӷ���ʽ��____________________________________________��
��3��Ϊ��֤���������ǿ�ڵ⣬�������IJ�����������________________________________��
��4��������ʵ���Ŀ����_________________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��������أ�K2FeO4����һ�ּ�������������������һ������Ͷ��ˮ���������������������£�

��1��д����ҵ����ȡCl2�Ļ�ѧ����ʽ_____________________________________��
��2��������ӦҺI���м���KOH�����Ŀ���Ǣ�_________________�����ṩ���Ի�����
��3��д��Fe(NO3)3��Һ�����KClO��Һ��Ӧ�Ļ�ѧ����ʽ��______________________��
��4��K2FeO4����Ϊ���Ͷ��ˮ��������ԭ���ǣ�
��_____________________����_________________________��
��5��������ӦҺII���з����K2FeO4����Ʒ��_________________��д��ѧʽ����
��6���ù���ÿ�õ�1.98 kg K2FeO4������������Cl2�����ʵ���Ϊ_______mol��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com