
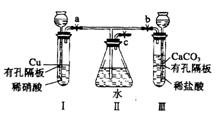
����ͼʵ��װ�ã�����a��b��cΪ���ɼС�
����1���������a��c���ر�b����I�з�Ӧ�����ӷ���ʽΪ________________________������2�������I���е�ϡ���ỻ��Ũ���ᣬ����a��c���ر�b����һ����۲죬���Կ���II�����ˮ�еĵ��ܿ�������ð���������������______ɫ��Һ��������Ϊ_____ɫ��
��3�������⣨1����ʵ���У�ҪʹII�������ʼ�ձ�����ɫ��Ӧ�ȹر�_____����a��b����ͬ��������____��c��ʹ������CO2�����ž�II��Ŀ������ٹر�_____��_____��
��4������32gͭ����ʢ��150mLһ��Ũ�������I��ʹ֮ǡ����ȫ��Ӧ��������NO2��NO��������ڱ�״���µ����Ϊ11.2L����NOΪ__________mol��ԭ������Һ��Ũ��Ϊ___________mol![]() L-1��
L-1��

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
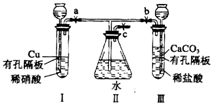 ����ͼʵ��װ�ã�����a��b��cΪ���ɼУ�
����ͼʵ��װ�ã�����a��b��cΪ���ɼУ��鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

| ||
| ||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
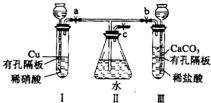 ����ͼʵ��װ�ã�����a��b��cΪ���ɼУ�
����ͼʵ��װ�ã�����a��b��cΪ���ɼУ��鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ��ɽ��ʡ�������ϣ����л�ѧ�Ծ��������棩 ���ͣ������
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com