
����Ŀ������þ��(Mg2B2O5��H2O����Fe2O3����)��ȡ����(H3BO3)������������¡�
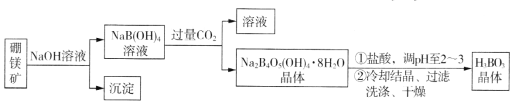
ͬ���������⣺
(1)��������Ҫ�ɷ�Ϊ____________________(�ѧʽ)��
(2)д������Na2B4O5(OH)4��8H2O�Ļ�ѧ����ʽ_________________________________��
(3)����H3BO3����ϴ�Ӹɾ��IJ�����______________________________��
(4)��֪��
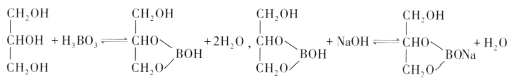
ʵ�������ô�ԭ���ⶨ������Ʒ�����������������ȷ��ȡ0.3000g��Ʒ����ƿ�У�����������ͼ���ʹ�����ܽⲢ��ȴ������1��2�η�̪��Һ��Ȼ����0.2000mol��L-1NaOH����Һ�ζ����յ㣬����NaOH��Һ22.00mL��
�ٵζ��յ������Ϊ________________________��
�ڸ�������Ʒ�Ĵ���Ϊ_________________��(����1λС��)��
(5)���NaB(OH)4��Һ�Ʊ�H3BO3�Ĺ���ԭ������ͼ��
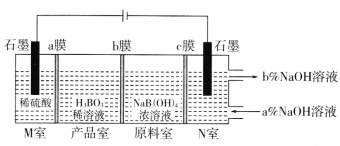
��bĤΪ________����Ĥ(������������������������)��������ÿ����1molH3BO3�������ҹ�����__________L����(��״��)��
��N���У����ںͳ���NaOH��Һ��Ũ�ȣ�a��_________b��(����>������<��)��
���𰸡�Mg(OH)2��Fe2O3 4NaB��OH��4+2CO2+3H2O=Na2B4O5��OH��48H2O��+2NaHCO3 ȡ���һ��ϴ��Һ�������Թ��У��μ������ữ����������Һ��������������˵��ϴ�Ӹɾ� ��Һ����ɫ��Ϊdz��ɫ���Ұ�����ڲ���ɫ 90.9 ������ 16.8 <
��������
��þ��������������Һ��Ӧ�����˳�ȥ����Mg��OH��2��Fe2O3��NaB��OH��4��Һ��ͨ������Ķ�����̼���õ�Na2B4O5��OH��48H2O��ΪNaHCO3�����˷��룬�������������С�����ᣬ���ϸ��ֽⷴӦ��ǿ���������ԭ������������ܽ�Ƚ�С����Na2B4O5��OH��48H2O���������ᷴӦ�õ����ᣬ��ȴ�ᾧ�����ˡ�ϴ�ӡ�����õ�����(H3BO3)���塣
(1)þ���ӿ�������������þ���������������������������Ʒ�Ӧ��
(2)��Ӧ��NaB��OH��4����CO2��������Na2B4O5(OH)4��8H2O��̼�����ƣ�д����ѧ����ʽ��
(3)ȡ���һ��ϴ��Һ���������������飻
(4) �ٸ��ݷ�̪�����ɫ�����������жϣ�
�����ù�ϵʽ�����м��㣻
(5)M������������ʧ���ӣ������Ӿ���aĤ�����Ʒ�ң�aĤΪ�����ӽ���Ĥ��ԭ������B��OH��4-ͨ��bĤ�����Ʒ����M�ҽ����H+��Ӧ����H3BO3��bĤΪ�����ӽ���Ĥ��ԭ����Na+����cĤ����N�ң�cĤΪ�����ӽ���Ĥ��N�������ӵõ�����������������������Ũ��������M����������������ˮ��N�������������ƺ��������ݴ˷�����
(1) ��þ�����������Ʒ�Ӧ��þ��������������þ������Fe2O3�����������Ʒ�Ӧ����˳�������Ҫ�ɷ�ΪMg(OH)2��Fe2O3��
�𰸣�Mg(OH)2��Fe2O3
(2) ��Ӧ��NaB��OH��4����CO2��������Na2B4O5(OH)4��8H2O��̼�����ƣ���ѧ����ʽΪ4NaB��OH��4+2CO2+3H2O=Na2B4O5��OH��48H2O��+2NaHCO3��
�𰸣�4NaB��OH��4+2CO2+3H2O=Na2B4O5��OH��48H2O��+2NaHCO3
(3)����H3BO3����ϴ�Ӹɾ��IJ�����ȡ���һ��ϴ��Һ�������Թ��У��μ������ữ����������Һ��������������˵��ϴ�Ӹɾ���
�𰸣�ȡ���һ��ϴ��Һ�������Թ��У��μ������ữ����������Һ��������������˵��ϴ�Ӹɾ�
(4)�ٵζ��յ������Ϊ��Һ����ɫ��Ϊdz��ɫ���Ұ�����ڲ���ɫ��
�𰸣���Һ����ɫ��Ϊdz��ɫ���Ұ�����ڲ���ɫ
��H3BO3��NaOH
1mol 1mol
n��H3BO3��0.2000mol/L��22.00��10-3L
��n��H3BO3��=0.0044mol
m��H3BO3��= n��H3BO3����M��H3BO3��=0.0044mol��62g/mol=0.2728g
����Ϊ![]() ��100%=90.9%
��100%=90.9%
(5)M������������ʧ�������������������Ӿ���aĤ�����Ʒ�ң�aĤΪ�����ӽ���Ĥ��ԭ������B��OH��4��ͨ��bĤ�����Ʒ����M�ҽ����H+��Ӧ����H3BO3��bĤΪ�����ӽ���Ĥ��ԭ����Na+����cĤ����N�ң�cĤΪ�����ӽ���Ĥ��N�������ӵõ�����������������������Ũ��������
�������������֪bĤΪ�����ӽ���Ĥ����ΪH++ B��OH��4��=H3BO3+H2O�����ת��1mol��������1mol H3BO3���й�ϵʽ
1mole-��1/4O2��M�ң���1mol H3BO3��1/2H2��N�ң�
������ÿ����1molH3BO3�������ҹ����ɣ�1/2+1/4��mol��22.4L/mol=16.8L����(��״��)��
�������������֪N���У����ںͳ���NaOH��Һ��Ũ�ȣ�a��<b����
�𰸣������� 16.8 <



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ʵ������֤�����������̼������Ӧ����ȷ��������װ����ͼ��ʾ(��֪��![]() ��Һ��
��Һ��![]() �ܲ�����ɫ��
�ܲ�����ɫ��![]() )������˵���������( ����)
)������˵���������( ����)
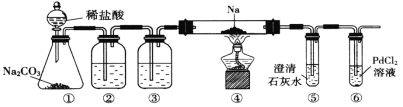
A.װ�âٵ�������������ȡ![]() ��
��![]() ������
������
B.װ�â���ʯ��ˮ����Ǻ��ٵ�ȼ�ƾ���
C.װ�âڢ��зֱ�ʢװ����![]() ��Һ��Ũ
��Һ��Ũ![]()
D.װ�â����к�ɫ�����������ķ�Ӧ��![]()
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����ʵ����ͺ���Ԫ�صĻ��ϼ����о��������ʵ�������Ҫά�ȡ���ͼΪ���䲿�ֻ�������������άͼ���� ����ͼʾ�ش��������⣺
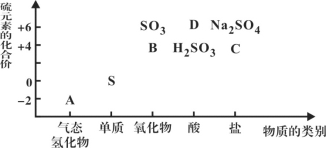
(1)ͼ��A��B��C��D���������У����ڷǵ���ʵ���__________(�û�ѧʽ��ʾ)��
(2)����Bͨ����ɫʯ����Һ��������______________________________________��
FeCl3��Һ(����)��ͨ��B�����ӷ���ʽΪ___________________________��
(3)C�ڷ�Ӧ�мȿ������������ֿ�����ԭ����������������ʱ����������ɱ���ԭΪ____(����)��
A��Na2S B��S C��H2SO3 D��Na2SO4 E��H2SO4
(4)����Ԫ�ػ��ϼ۷��������ʾ��������Ժͻ�ԭ�ԡ����һ��˵�������������(�û�ѧ����ʽ��ʾ)_______________________________��
(5)��A��SO2��ϣ������ɵ���ɫ���塣�÷�Ӧ�����������뻹ԭ���������֮��Ϊ__________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ϩ����Ҫ�Ļ���ԭ�ϣ�����ϩΪԭ���ڲ�ͬ�����¿ɺϳ��������ʣ���������δ�������

������Ҫ��д����
��1������ϩ�Ľṹ��ʽ��_____________����ȩ�Ľṹ��ʽ��_____________��
��2����Ӧ�ٵĻ�ѧ����ʽ��________________________________����Ӧ������___________��
��3����Ӧ�۵Ļ�ѧ����ʽ��________________________________����Ӧ������___________��
��4����Ӧ�ݵĻ�ѧ����ʽ��________________________________����Ӧ������___________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��CH4��������CO2�����ɵõ�����CO�Ļ���ԭ�ϡ��ش��������⣺
��1��CH4��������CO2�Ĵ�ת����ͼ��ʾ��
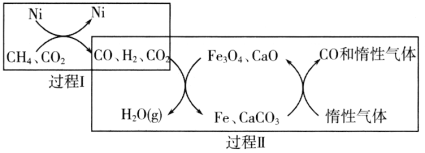
����֪��ط�Ӧ�������仯��ͼ��ʾ��

���̢���Ȼ�ѧ����ʽΪ________��
�ڹ����������̢��˵������ȷ����________������ţ���
a��ʵ���˺�̼�����뺬�����ʵķ���
b���ɱ�ʾΪCO2��H2��H2O��g����CO
c��COδ���뷴Ӧ
d��Fe3O4��CaOΪ�����������˷�Ӧ�Ħ�H
�������������䣬�ڲ�ͬ�������������£���ӦCH4��g����CO2��g����2CO��g����2H2��g��������ͬʱ���CH4��ת�����淴Ӧ�¶ȵı仯��ͼ��ʾ��a����������״̬________����ǡ����ǡ���ƽ��״̬��b��CH4��ת���ʸ���c�㣬ԭ����________��
��2����һ�����ܱ������У�CH4��CO2�ķ�ѹ�ֱ�Ϊ20kPa��25kPa������Ni������Al2O3������������1123Kʹ�䷢����ӦCH4��g����CO2��g����2CO��g����2H2��g����

���о�����CO���������ʦԣ�CO����1.3��10��2��p��CH4����p��CO2��mol��g��1��s��1��ijʱ�̲��p��CO����20kPa����p��CO2����________kPa���ԣ�CO����________mol��g��1��s��1��
�ڴﵽƽ�������ϵѹǿ����ʼʱ��1.8������÷�Ӧ��ƽ�ⳣ���ļ���ʽΪKp��________��kPa��2�����ø����ʵķ�ѹ�������ʵ���Ũ�ȼ��㣩
��3��CH4��������CO2�õ���CO��ż����Ӧ���Ƶò��ᣨH2C2O4���������£���ijŨ�ȵIJ�����Һ�м���һ��Ũ�ȵ�NaOH��Һ��������Һ��![]() �����ʱ��Һ��pH��________������֪������H2C2O4��Ka1��6��10��2��Ka2��6��10��5��lg6��0.8��
�����ʱ��Һ��pH��________������֪������H2C2O4��Ka1��6��10��2��Ka2��6��10��5��lg6��0.8��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����֪AΪ����ɫ���壬R�ǵؿ��к������Ľ���Ԫ�صĵ��ʣ�TΪ������ ʹ����㷺�Ľ������ʣ�D�Ǿ��д��Եĺ�ɫ���壬C��F����ɫ��ζ�����壬H�ǰ�ɫ������
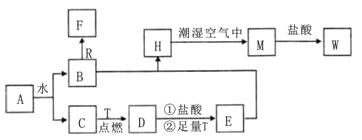
��1������A�Ļ�ѧʽΪ ________��F��ѧʽΪ ________��
��2��B��R����Һ�з�Ӧ����F�����ӷ���ʽΪ__________________________��
��3��H�ڳ�ʪ�����б��M��ʵ��������________����ѧ����ʽΪ______________��
��4��A��ˮ��Ӧ����B��C�Ļ�ѧ����ʽΪ__________________________
��5��ӡˢ��ҵ����ӡˢ��·�壨����ͭ����ʱ��Ҫ��W��Һ����Ϊ����ʴҺ����д���÷�Ӧ�����ӷ���ʽ______________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����10�֣�ijͬѧ����ˮ�ʼ��վ����480 mL 0.5 mol��L��1NaOH��Һ�Ա�ʹ�á�
��1����ͬѧӦѡ��________mL������ƿ��
��2���������������ͼ��ʾ��
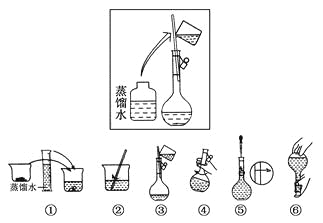
����ͼ����Ӧ����ͼ�е�________(��ѡ����ĸ)֮�䡣
A�������� B�������� C��������
��3����ͬѧӦ��ȡNaOH����________g��������Ϊ23.1 g���ձ�����������ƽ�ϳ�ȡ����NaOH����ʱ�����ڸ�����ѡȡ����������С________(����ĸ)��������ͼ��ѡ������ȷ��ʾ����λ�õ�ѡ��________(����ĸ)��
���� ������
a | b | c | d | e | |
�����С/g | 100 | 50 | 20 | 10 | 5 |
��4�����в�����������Һ��Ũ�ȴ�С�к�Ӱ�죿
��ת������Һ��δϴ�Ӳ��������ձ���Ũ�Ȼ�________(����ƫ��������ƫС��������Ӱ��������ͬ)
������ƿ��ԭ������������ˮ��Ũ�Ȼ�________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��I. ij���淴Ӧ��ij���Ϊ2L���ܱ������н��У��ڴ�0~3min�����ʵ����ı仯�����ͼ��ʾ��A��B��C��Ϊ���壩
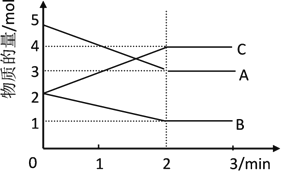
��1���÷�Ӧ�Ļ�ѧ����ʽΪ______
��2����Ӧ��ʼ��2minʱ��B��ƽ����Ӧ����Ϊ______
��3����˵���÷�Ӧ�Ѵﵽƽ��״̬����______
A. c(A)= c(B)= c(C) B. ������ѹǿ���ֲ��� C . v��(A)= v��(C) D. c(C)���ٱ仯
��4����ͼ���A��ƽ��ʱ��ת����Ϊ______
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ˮ����Ԫ����Br��ʽ���ڣ���ҵ���ÿ����������Ӻ�ˮ����ȡ��Ĺ���������ͼ��ʾ��
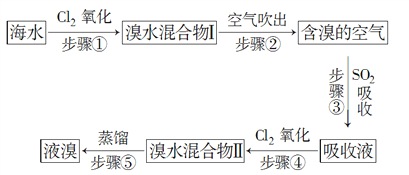
(1)����ٷ�Ӧ�����ӷ���ʽ��____________________������۷�Ӧ�Ļ�ѧ����ʽ��___________________��
(2)�������Ͽ��ǣ���������Ҳ������Br2����___________(����ĸ)��
A��NaOH�������� B��FeCl2
C��Na2SO3 D��H2O
(3)���������������________������Ӧ������2 mol HBr��������________mol SO2��
(4)����������Ӧ�ж�SO2��Cl2��Br2����������������ǿ������˳����________________��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com