
����Ŀ����Ԫ�ؿ��γɶ��ֻ�����ڹ�ҵ�����о�����Ҫ��ֵ�� ��ش��������⣺
��1����֪��1mol H��H ����1mol N��H���� 1mol N��N���ֱ���Ҫ��������436 kJ��391 kJ��946 kJ���Ҹ÷�ӦΪ���淴Ӧ����N2��H2��Ӧ�ϳ�NH3���Ȼ�ѧ����ʽΪ_________��
��2��һ���¶��£���һ������N2��H2����̶�������ܱ������н��кϳɰ���Ӧ��
������������˵���ÿ��淴Ӧ�ﵽ��ѧƽ��״̬����___________
A.������������ܶȲ��� B.c(N2)��c(H2)��c(NH3)=1��3��2
C.�����ڵ�ѹǿ���� D.3v��(H2) =2v��(NH3)
E. �����������ƽ����Է�����������
F. ��ͬʱ������3molH-H�����ѣ���6mol N-H���γ�
�ں��º�ѹ�����£�Ϊ��ߺϳɰ���Ӧ��N2��H2�������ʣ����Բ��õķ�����________________��
��3��һ���¶��£�2L�ܱ������г���0.40 mol N2O4��������Ӧ��N2O4(g)![]() 2NO2(g)��һ��ʱ���ﵽƽ�⣬����������£�
2NO2(g)��һ��ʱ���ﵽƽ�⣬����������£�
ʱ�䣯s | 20 | 40 | 60 | 80 | 100 |
c(NO2)/(mol/L) | 0.12 | 0.20 | 0.26 | 0.30 | 0.30 |
��20s�ڣ�v(NO2)=___________�����¶��·�Ӧ�Ļ�ѧƽ�ⳣ����ֵΪ_________��
�������¶�ʱ��������ɫ���������Ӧ��_________(��������������������)��Ӧ��
����ͬ�¶��£�����ʼ��������г���0.40 mol NO2����ﵽƽ���: c(NO2)_____0.15 mol��L-1(����>���� ��=������<��)
���𰸡�N2(g)+3H2(g)![]() 2NH3(g) ��H��-92 kJ��mol��1CE��ʱ��NH3��ȴҺ�������ȥ����ʱ����������������ѭ������0.006 mol��L-1��s-11.8���ȣ�
2NH3(g) ��H��-92 kJ��mol��1CE��ʱ��NH3��ȴҺ�������ȥ����ʱ����������������ѭ������0.006 mol��L-1��s-11.8���ȣ�
��������
(1)�ڷ�ӦN2+3H22NH3�У�����3molH-H����1molN��N�������յ�����Ϊ��3��436kJ+946kJ=2254kJ������2molNH3ʱ���γ�6molN-H�����ų�������Ϊ��6��391kJ=2346kJ�����յ������٣��ų��������࣬�÷�ӦΪ���ȷ�Ӧ���ų�������=2346kJ-2254kJ=92kJ����N2��H2��Ӧ����NH3���Ȼ�ѧ����ʽΪ��N2(g)+3H2(g)2NH3(g)��H=-92kJmol-1���ʴ�Ϊ��N2(g)+3H2(g)2NH3(g)��H=-92kJmol-1��
(2)��A���÷�Ӧ�л�����������������ݻ�Ϊ��ֵ������������ܶ�ʼ�ղ��䣬���ܸ��ݻ��������ܶ��ж�ƽ��״̬����A����B��c(N2)��c(H2)��c(NH3)=1��3��2�����жϸ���ֵ�Ũ���Ƿ�����仯�������ж�ƽ��״̬����B����C���÷�ӦΪ���������С�ķ�Ӧ��ѹǿΪ�������������ڵ�ѹǿ���䣬�������淴Ӧ������ȣ��÷�Ӧ�ﵽƽ��״̬����C��ȷ��D.��Ӧ����ƽ��ʱ��2v��(H2)=3v��(NH3)����D����E. �÷�Ӧ����������ʵ�����С�ķ�Ӧ����������ʵ���Ϊ����������![]() =
=![]() �������������ƽ����Է����������䣬˵������������ʵ������䣬�ܹ�˵���ﵽƽ��״̬����E��ȷ��F����ͬʱ������ 3molH-H �����ѣ��� 6molN-H ���γɣ���ʾ�Ķ�������Ӧ���ʣ����ж����淴Ӧ�����Ƿ���ȣ���F���ʴ�Ϊ��C E��
�������������ƽ����Է����������䣬˵������������ʵ������䣬�ܹ�˵���ﵽƽ��״̬����E��ȷ��F����ͬʱ������ 3molH-H �����ѣ��� 6molN-H ���γɣ���ʾ�Ķ�������Ӧ���ʣ����ж����淴Ӧ�����Ƿ���ȣ���F���ʴ�Ϊ��C E��
�� ���º�ѹ�����£�Ϊ��ߺϳɰ���Ӧ��N2��H2�������ʣ���ƽ����������Ӧ�����ƶ������Բ��õķ����м�ʱ��NH3��ȴҺ�������ȥ����ʱ����������������ѭ�����õȣ��ʴ�Ϊ����ʱ��NH3��ȴҺ�������ȥ����ʱ����������������ѭ�����ã�
(3)��20sʱNO2��Ũ��Ϊ0.12mol/L����20s���ö���������ʾ��ƽ����Ӧ����v(NO2)=![]() =0.006molL-1s-1����ʼʱc(N2O4)=
=0.006molL-1s-1����ʼʱc(N2O4)=![]() =0.2mol/L�����ݱ������ݣ�ƽ��ʱc(NO2)=0.3mol/L����c(N2O4)=0.2mol/L-0.15mol/L=0.05mol/L����ѧƽ�ⳣ��K=
=0.2mol/L�����ݱ������ݣ�ƽ��ʱc(NO2)=0.3mol/L����c(N2O4)=0.2mol/L-0.15mol/L=0.05mol/L����ѧƽ�ⳣ��K=![]() =1.8���ʴ�Ϊ��0.006 molL-1s-1��1.8��
=1.8���ʴ�Ϊ��0.006 molL-1s-1��1.8��
�������¶�ʱ��������ɫ���˵�����º�ƽ�������ƶ���������ӦΪ���ȷ�Ӧ���ʴ�Ϊ�����ȣ�
����ͬ�¶��£�����ʼ��������г���0.40 mol NO2���൱�ڿ�ʼ��������г���0.20 mol N2O4����������������䣬���൱�ڼ�Сѹǿ��ƽ�������ƶ��������ﵽƽ���: c(NO2)����ԭ����һ�룬��c(NO2)��0.15 mol��L-1���ʴ�Ϊ������



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���йؼ�������ʵ�����������ǣ� ��
A.��˵���������ӣ���������ۡ��е㽵��
B.��˵���������ӣ���������ܶ�����
C.���������ȼ�����ɹ������
D.�������ˮ��Ӧ���ɼ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����һ�ݻ�������ܱ������г���һ����A��B���������·�Ӧ��xA(g)��2B(s) ![]() yC(g)����H<0��һ�������£�������A��C�����ʵ���Ũ����ʱ��仯��������ͼ��ʾ����ش��������⣺
yC(g)����H<0��һ�������£�������A��C�����ʵ���Ũ����ʱ��仯��������ͼ��ʾ����ش��������⣺

��1����A��Ũ�ȱ仯��ʾ�÷�Ӧ��0��10 min�ڵ�ƽ����Ӧ����v(A)��______________________��
��2������ͼʾ��ȷ��x��y��________��
��3��0��10 min������ѹǿ________(���������䡱��С��)��
��4���Ʋ��10 min�������߱仯�ķ�Ӧ����������______________________����16min�������߱仯�ķ�Ӧ����������________________________��
�ټ�ѹ��������A��Ũ�ȡ� ������C������ �����¢ݽ��¡� �Ӵ���
��5����ƽ����ƽ�ⳣ��ΪK1��ƽ���ƽ�ⳣ��ΪK2����K1________K2(�����������������)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����ǵ�IIIA��Ԫ�أ��������ڼ���������������ַǽ�����Ӧ��ijͬѧ��������������������Ӧ�Ʊ����Ȼ�����֪BC13�ķе�Ϊ12.5 �� ���۵�Ϊ-107.3 �棬��ˮ���ҷ�Ӧ��������������ᡣ��ͬѧѡ����ͼ��ʾ�IJ���װ�ã������ظ�ѡ�ã�����ʵ�飬��ش��������⣺
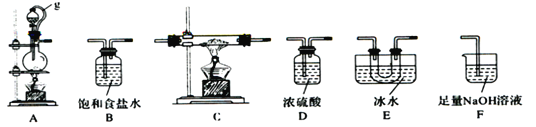
��1��A�з�Ӧ�����ӷ���ʽΪ__________________��
��2��ͼ��g�ܵ�������______________________________________��
��3��װ�õ�����˳������ΪA�� �� �� ��E��D��F��____________��E��Fװ�ü�����Dװ�õ�������____________________________________________________________��
��4��ֹͣʵ��ʱ����ȷ��ʵ�������______________________________________________________________________________________________________________��
��5��������(H3BO3)ΪһԪ���ᣬ��������NaH2BO3Ϊ_____������Ρ�����ʽ�Ρ���ʽ�Ρ�����
��6��ʵ����ɺ�ijͬѧ��F�У���Һ����0.05mol/LNaC1O�� 0.05mol/LNaCl��0.1mol/LNa0H���μ�Ʒ����Һ��������Һ��ɫ�������ʵ��̽����Һ��ɫ��ԭ�����ڱ��пո��������ݣ����ʵ�鷽����
ʵ����� | 0.1mol/LNaClO��Һ/mL | 0.1mol/LNaCl��Һ/mL | 0.2mol/LNaOH��Һ/mL | H2O /mL | Ʒ�� ��Һ | ���� |
�� | 5.0 | 0 | 0 | x | 4�� | �Ͽ���ɫ |
�� | 0 | 5.0 | 5.0 | 0 | 4�� | ����ɫ |
�� | 5.0 | 0 | 5.0 | 0 | 4�� | ������ɫ |
��x=_______�����ۣ�________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���绯ѧ���������������ڼ�����NH3�ĺ������乤��ԭ��ʾ��ͼ���¡�

����˵������ȷ����
A. O2�ڵ缫b�Ϸ�����ԭ��Ӧ
B. ��Һ��OH+��缫a�ƶ�
C. �����ĵ缫��ӦʽΪ��2NH3-6e-+6OH-=N2+6H2O
D. ��Ӧ���ĵ�NH3��O2�����ʵ���֮��Ϊ4��5
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��0.01mol�Ȼ���(CrCl3��6H2O)��ˮ��Һ���ù�����AgNO3����������0.01mol AgCl���������Ȼ����������(����)
A. [Cr(H2O)6]Cl3 B. [Cr(H2O)5Cl]Cl2��H2O
C. [Cr(H2O)4Cl2]Cl��2H2O D. [Cr(H2O)3Cl3]��3H2O
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���⻯���ƣ�NaAlH4����һ���������ʴ�����ϣ���������Ti��NaAlH4��150��ʱ���⣬��170�桢15.2MPa���������ظ����⡣NaAlH4����AlCl3��NaH���ʵ������ºϳɡ�NaAlH4�ľ����ṹ��ͼ��ʾ��

��1����̬Tiԭ�ӵ��۵��ӹ����ʾʽΪ_______��
��2��AlH4-�Ŀռ乹��Ϊ_______________������ԭ��Al�Ĺ���ӻ���ʽΪ________��
��3��AlCl3��178��ʱ����������������Է�������ԼΪ267���������ӵ��ṹʽΪ________________��������λ������
��4��NaH���۵�Ϊ800�棬�������л��ܼ�NaH����____���壬�����ʽΪ_____________��
��5��NaAlH4�����У���Na+�����ҵȾ��AlH4-��_____����NaAlH4������ܶ�Ϊ________g��cm-3���ú�a�Ĵ���ʽ��ʾ������NaAlH4�������Ĵ���Na+��Li+ȡ�����õ��ľ���Ϊ__________���ѧʽ����
��6��NaAlH4���������Ϊ��ÿ3��AlH4-�У���2���ֱ��ͷų�3��Hԭ�Ӻ�1��Alԭ�ӣ�ͬʱ���Alԭ������ڵ�Naԭ��ת�Ƶ����ͷŵ�Alԭ�����µĿ�λ���γ��µĽṹ�����ֽṹ�仯�ɱ������չ���������壬�Ӷ��ͷų���������������̿��û�ѧ����ʽ��ʾΪ____________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����б仯�У�������ͨ��һ����Ӧʵ�ֵ��ǣ�������
A.SiO2��Na2SiO3B.SiO2��H2SiO3C.SiO2��SiD.Si��SiCl4
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��
�������ڵĹ���Ԫ���ڹ�ҵ��ũҵ����ѧ�����Լ���������ȷ�������Ҫ���á�����Ni-Cr-Fe�Ͻ��dz��õĵ���Ԫ�����ϡ���ش�:
(1) ��̬Niԭ�Ӻ�������Ų�ʽΪ________���ڶ������л�̬ԭ��δ�ɶԵ�������Ni��ͬ�ҵ縺�Դ��Ԫ��Ϊ________ ��
(2) ����Ni����CO�����������ȣ�������ɫ�ӷ���Һ̬Ni(CO)4���÷��ӳ��������幹�͡����ƶ�Ni(CO)4�ľ�������Ϊ_____��Ni(CO)4 �� ��������_____ (��ѡ����ĸ) �С�
A.ˮ B.���Ȼ�̼ C.�� D.��������Һ
(3) FeO��NiO ������r(Ni2+)��r(Fe2+)�ֱ�Ϊ69pm ��78pm,���۵�NiO__FeO(����>"����<��)��ԭ��Ϊ_________ ��
(4) ��Ѫ����һ�������仯ѧʽΪK4[Fe(CN)6]��3H2O���������������Ļ�ѧʽΪ_________����Ѫ����Һ��ϡ�������ʱ������������ԭ��Ӧ�����������κ�һ��������廥Ϊ�ȵ��������̬������÷�Ӧ�Ļ�ѧ����ʽΪ_________ ��
(5) ����K2Cr2O7�������������ӣ����������ӺͶ�����������20��ˮ�е��ܽ��֮��Ϊ0.39����ԭ��Ϊ________��
(6) �ڸ��Ĺ������У�SiO44-����������ͼ(a)ͨ�����ö��������ӿ��γɵ�״����״����״���Ǽ���״�Ĵ�ṹ��ʽ��ͼ(b)Ϊһ����״�ṹ�Ķ����������й�ԭ�ӵ��ӻ���ʽΪ_______���仯ѧʽΪ________��
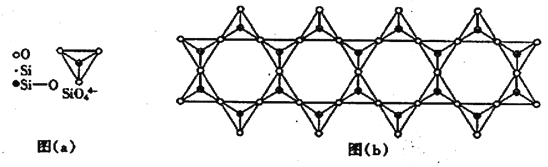
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com