
 ��һ������Ԫͬ�����ؾ�ϵ�д�
��һ������Ԫͬ�����ؾ�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
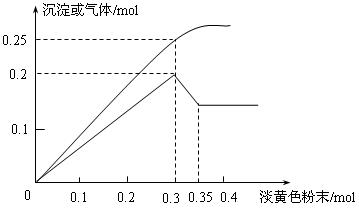
 ���
��� �ǡ�����������ԭ��Ӧ��д��̼�����Ƶ�һ����; ��
�ǡ�����������ԭ��Ӧ��д��̼�����Ƶ�һ����; ���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
 4X��SiC��3C��
4X��SiC��3C���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����

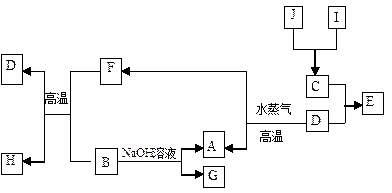
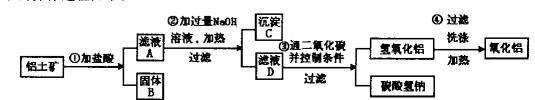
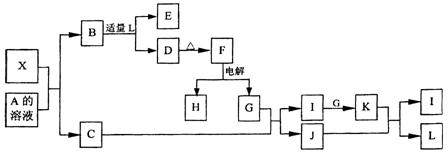
 (1)д���������ʵ�����A ��G ��
(1)д���������ʵ�����A ��G �� (2)д�����з�Ӧ�Ļ�ѧ����ʽ��
(2)д�����з�Ӧ�Ļ�ѧ����ʽ�� ��B+F��D+H�� ��
��B+F��D+H�� �� ��D��A+F�� ��
��D��A+F�� �� ��B��A+G�� ��
��B��A+G�� �� (3)������J�����ữ��H2O2����Һ�У�J�ܽ���������+2�����ӣ��÷�Ӧ�����ӷ���ʽ��
(3)������J�����ữ��H2O2����Һ�У�J�ܽ���������+2�����ӣ��÷�Ӧ�����ӷ���ʽ��  ��
���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
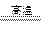 2MgO+Si(�����к���һ�����Ķ�������)��ͬʱ�и���Ӧ������2Mg+Si
2MgO+Si(�����к���һ�����Ķ�������)��ͬʱ�и���Ӧ������2Mg+Si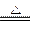 Mg2Si��Mg2Si������Ѹ�ٷ�Ӧ����SiH4(����)��SiH4�ڳ�������һ�ֲ��ȶ����ֽ�����塣
Mg2Si��Mg2Si������Ѹ�ٷ�Ӧ����SiH4(����)��SiH4�ڳ�������һ�ֲ��ȶ����ֽ�����塣
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��һ���г������� |
| B��һ���н���������? |
| C�����һ���г������ɣ���������ų�? |
| D����Һ���һ�����壬��������ų�? |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��Al2O3��MgO��SiO2���Dz�����ˮ�Ĺ��� |
| B��Al2O3��MgO��SiO2�����������Ӿ��� |
| C��Al2O3��MgO��SiO2���������� |
| D��Al2O3��MgO��SiO2���кܸߵ��۵� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
 ����ȷ���� �� ��
����ȷ���� �� ��| A�����յõ�����Һ��ֻ��NaCl���� |
| B�����յõ�7��8g���� |
| C����״���£���Ӧ�����еõ�6��72L������ |
| D�����յõ�����Һ��c��Na+����1��5mol/L |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com