
| A��25��ʱ��pH=7��CH3COOH��CH3COONa�Ļ��Һ������Ũ�ȵĴ�С˳��Ϊ��c��Na+����c��CH3COO-����c��H+��=c��OH-�� |
| B��25��ʱ��0.1mol/L NaHA��ҺpH=3������Һ��ijЩ����Ũ�ȴ�С˳��Ϊ��c��HA-����c��H+����c��H2A����c��A2-�� |
| C��25��ʱ����10mL pH=a��������100mL pH=b ��Ba��OH��2��Һ��Ϻ�ǡ���кͣ���a+b=13 |
| D��25��ʱ��Ka��HF��=3.6��10-4��Ka��CH3COOH��=1.75��10-5��0.1mol/L ��NaF��Һ��0.1mol/L ��CH3COOK��Һ��ȣ�c��Na+��-c��F-����c��K+��-c��CH3COO-�� |



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A�������ص���Ҫ�����ǵ���ͷ����pH��ʹ֮�ﵽ���˵����� |
| B�������������ס�����������������pH�������� |
| C������1mol/L KI��Һ��0.1mol/L H2SO4��Һ�͵�����Һ���������ޣ����Ϳ���̽���¶ȶԻ�ѧ��Ӧ���ʵ�Ӱ�� |
| D��������Mg��OH��2�����������Ȼ����Һ��ϣ����������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ��������ѧ���õ�һ�����ᣮ
��������ѧ���õ�һ�����ᣮ�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| 1 |
| 3 |
| A���٢ڢۢܢޢ�� |
| B���ڢܢݢޢߢ� |
| C���ۢܢݢޢ�� |
| D���٢ڢܢ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A������CO2���ŷţ����Լ�������IJ��� |
| B������SO2���ŷţ����ԴӸ������������� |
| C�����칬һ�š�ʹ�õ�̼��ά����һ�������л��߷��Ӳ��� |
| D����CO2�ϳɾ�̼�����ɽ������ϣ�����ʵ�֡�̼����ѭ������ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��2NO+O2?2NO2 |
| B��N2O4?2NO2 |
| C��Br2��g��+H2?2HBr |
| D��6NO+4NH3?5N2+3H2O |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A | B | C | D | |
ͼʾ |
 |
 |
 |
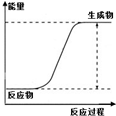 |
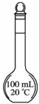
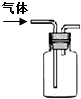
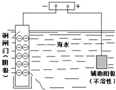
| ˵�� | ��������������һ��������������Һ | ��װ�ÿ����ռ��������� | ��װ�ÿɱ�����բ�Ų�����ʴ | �û�ѧ��ӦΪ���ȷ�Ӧ |
| A��A | B��B | C��C | D��D |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

| A��һ��ʱ������ձ���c��Zn2+����c��Cu2+������С |
| B��ԭ��ع���ʱ��Cu�缫�������ӣ�����������Ӧ |
| C��ԭ��ع���ʱ���ܷ�ӦΪZn+Cu2+�TZn2++Cu |
| D��������װ�к��Ȼ��ص���֬���������Ǵ��ݵ��� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com