ij������;�㷺�������������Լ���ýȾ��������������ԭ�ϣ����ⶨ��������Ԫ�أ�Ħ������Ϊ482g/mol��Ϊ��һ��ȷ��������ɣ�ij��ѧ��ȤС����������ʵ�飺
��ȡ48.20g����������ˮ�����100mL��Һ��������Һ���ػ�ɫ��
��ȡ������Һ50mL���Թ��У�����������0.1mol/L NaOH��Һ�������ȣ����������徭�����ͨ��Ũ�����У�Ũ��������0.85g�������ĺ��ɫ�����������ˡ�ϴ�ӡ����պ��4.00g���壮
����ȡ������Һ50mL���Թ��У�����������BaCl
2��Һ����������������İ�ɫ���� 23.30g��
��ش��������⣺
��1��ʵ����в�������ĵ���ʽ
��
��2�������ʵĻ�ѧʽΪ
�������йظ����ʵ���;��������
��
A����Ѫ�� B����ˮ�� C�����ӷ�ˮ�ļ���Լ� D������
��3����������Һ����μ�������������Һ������Ԫ����ȫ����ʱ�Ļ�ѧ��Ӧ����ʽ
��
��4����SO
2����ͨ������ʵ���Һ�п��Թ۲쵽��ʵ��������
��д���÷�Ӧ�����ӷ���ʽ
��
��5��Ϊ�˽�һ����֤����������Ԫ�صĻ��ϼۣ�ijͬѧ���������ʵ�鷽����ȡ�����������Թ��У���ˮ����ܽ⣬�μ����軯����Һ������Һ��Ѫ��ɫ������֤����������һ������Fe
3+��
�����۸�ʵ������Ƿ����
������������ߡ�����������������
��

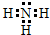 ���ʴ�Ϊ��
���ʴ�Ϊ��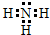 ��
��

 ǧ�������������ĩ�����Ծ�����ϵ�д�
ǧ�������������ĩ�����Ծ�����ϵ�д�